Abstract
Immune reconstitution therapy (IRT) is an emerging management concept for multiple sclerosis, whereby a short course of treatment provides long-lasting suppression of disease activity. “Cladribine tablets 10 mg” refers to a total cumulative dose of cladribine given over 2 years (henceforth referred to as cladribine tablets 3.5 mg/kg); it is a relatively new treatment option that is hypothesised to act as an IRT acting preferentially on the adaptive immune system. A randomised, 2-year, placebo-controlled trial (CLARITY) showed that treatment with cladribine tablets reduced indices of disease activity (relapses, lesions on magnetic resonance images, disability progression) and that this effect outlasted the pharmacologic effect of the treatment on the immune system (mainly a reduction in circulating B and T cells, with little effect on components of the innate immune system such as monocytes). CLARITY Extension, a 2-year extension to this trial, demonstrated durable efficacy, also in patients who received the standard 2-year course of cladribine tablets 3.5 mg/kg and were re-randomised to placebo for a further 2 years. Relative risk reductions for relapse rate with cladribine tablets 3.5 mg/kg were similar for patients with or without prior high disease activity. Reductions in disability progression with cladribine tablets 3.5 mg/kg were higher in patients with prior high relapse rates with or without prior treatment non-response. In this review, we describe the therapeutic profile of cladribine tablets 3.5 mg/kg and provide practical information on initiating this treatment option in the most appropriate patients.
Similar content being viewed by others
Immune reconstitution therapy (IRT) is an emerging management concept for relapsing multiple sclerosis (RMS), whereby a short course of treatment provides long-lasting suppression of disease activity. |
“Cladribine tablets 10 mg” refers to a total cumulative dose of cladribine given over 2 years (henceforth referred to as cladribine tablets 3.5 mg/kg); it is a relatively new treatment option, hypothesised to act as an IRT acting preferentially on the adaptive immune system. |
Results from randomised trials and from extensions to these trials have shown reductions in relapse rates that clearly outlasted the period of drug administration, consistent with an IRT-like mechanism. |
Treatment with cladribine tablets 10 mg is associated with a low rate of serious infections. |
The monitoring burden associated with the treatment is also low compared with other high-efficacy disease-modifying drugs for RMS. |
We describe the therapeutic profile of cladribine tablets 3.5 mg/kg and provide practical information on initiating this treatment in the most appropriate patients. |
Introduction
The introduction of immune reconstitution therapy (IRT) into the management of multiple sclerosis (MS) has the potential to provide durable efficacy over time without a need for sustained maintenance treatment [1, 2] In this review we describe the concept of pharmacologic IRT and summarise its practical application for the management of relapsing–remitting MS (RRMS), with special focus on “cladribine tablets 10 mg”, which refers to a total cumulative dose of cladribine given over 2 years (henceforth referred to as cladribine tablets 3.5 mg/kg). “cladribine tablets” is a new orally active disease-modifying drug (DMD) approved for use in RRMS in many countries, and cladribine tablets 10 mg is hypothesised to act as an IRT.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Brief Overview of Immune Reconstitution Therapy
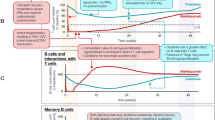
The application of an IRT induces a transient reduction in the number of circulating immune cells following a short course of treatment (described in more detail in subsequent sections) [1]. Accordingly, in theory, IRT may minimise the potential adverse consequences of long-term immunosuppression (especially infections and increased risk of malignancy), with a reduction in the rates of MS relapse and disease progression that outlasts the period of immune suppression [1]. Autologous hemopoietic stem cell therapy (aHSCT) is the most recognised form of IRT, and has been described as “rebooting” the immune system to provide long-lasting remission of otherwise refractory autoimmune and other diseases, including RRMS [3, 4]. While this treatment has become safer in recent years, it remains highly invasive, requiring complete ablation of the immune system, and it must be administered in specialist centres. Pharmacologic IRT has become an alternative option in recent years. Alemtuzumab (injectable anti-CD25 antibody) and cladribine tablets 3.5 mg/kg (oral administration) are currently available DMDs that have been suggested to act as IRTs [5,6,7].
Cladribine tablets 3.5 mg/kg is administered over 2 years (1.75 mg/kg/year) and given during two treatment weeks, one at the beginning of the first month and one at the beginning of the second month of each treatment year [5]. There is no requirement for further treatment in years 3 and 4 after the initiation of treatment according to the drug’s European labelling [5]. Alemtuzumab is given as a course of five doses of 12 mg/day on 5 consecutive days, followed by a second treatment course of 12 mg/day on 3 consecutive days 1 year later (up to two additional courses can be given if needed) [6]. Pre-treatment with corticosteroids is required before the administration of alemtuzumab for the first 3 days of any treatment course [6].
Other interventions may have the potential to act as IRTs. For example, the mechanism of ocrelizumab is consistent with a potential to act as an IRT; however, this agent is given continuously (chronic administration), albeit with a long (6-monthly) interval between successive maintenance doses. It is not known whether or not the efficacy of ocrelizumab outlasts the period of administration, and this agent is generally considered to be a continuous or maintenance therapy [8, 9]. Accordingly, the therapeutic profile of ocrelizumab is not described in detail here. Autologous haemopoietic stem cell transplantation has also been applied to people with MS, but this highly specialised intervention lies outside the scope of our review, which focuses on pharmacologic treatments.
Therapeutic Profile of Cladribine Tablets 3.5 mg/kg in Relapsing–Remitting Multiple Sclerosis
Effects on the Immune System
The administration of cladribine tablets induces a rapid and marked (about 50–85%) reduction in CD4+ T cells and to a lesser extent in CD8+ T cells [5]. Large reductions in CD19+ B cells are also seen, together with a reduction in CD16+/CD56+ natural killer cells, with the latter cells recovering faster than CD4+ T cells [5]. In one study, lymphocyte counts returned to normal in more than three-quarters of patients by 144 weeks after the initial administration of cladribine tablets. There was a lesser effect on other immune cell types, including monocytes and platelets, which typically remained within the normal range, a pharmacologic profile consistent with some degree of selectivity for the adaptive immune system [9].
Clinical Efficacy
The clinical efficacy of cladribine tablets 3.5 mg/kg was evaluated in 1326 patients with RRMS in the CLAdRIbine Tablets treating multiple sclerosis orallY (CLARITY) study, a randomised, placebo-controlled, 96-week clinical trial (Table 1) [10]. Briefly, compared to placebo, cladribine tablets 3.5 mg/kg was associated with lower relapse rates, a higher proportion of patients with no evidence of disease activity, less progression of disability and a reduction in disease activity as measured by magnetic resonance imaging (MRI) [10,11,12,13,14,15,16]. The reduction in disability progression was greater in patients with higher indices of disease activity at baseline than in those without higher baseline indices of disease activity [13]. The efficacy of cladribine tablets 3.5 mg/kg was similar in a range of other patient subgroups [12]. Reductions in relapse rates or the appearance of new MRI events (T1 gadolinium-enhancing lesions) with cladribine tablets 3.5 mg/kg versus placebo were comparable for the relatively higher or lower risk subgroups, defined according to the presence of higher disease activity at baseline, with or without disease activity on previous DMD treatment (treatment non-response; Fig. 1) [13]. Reductions in disability progression (6-month progression on the Expanded Disability Status Scale [EDSS]) and increases in NEDA (No Evidence of Disease Activity) status with cladribine tablets 3.5 mg/kg treatment were significantly greater in the higher disease activity subgroups (Fig. 1) [13]. Treatment with cladribine tablets 5.25 mg/kg was also evaluated in the CLARITY study, where it demonstrated a similar efficacy in MS but a greater potential for lymphopenia; accordingly, this dose is not used clinically and is not described in this article.

Adapted from Giovannoni et al. [13], first published by Sage on 2 May 2018, under the terms of the Creative Commons Attribution-NonCommercial 4.0 License
Effects of cladribine tablets 3.5 mg/kg (3.5 mg/kg cumulative dose over 2 years) vs. placebo on outcomes shown in subgroups with higher or lower multiple sclerosis disease activity at baseline in a post hoc analysis from the CLARITY (CLAdRIbine Tablets treating multiple sclerosis orallY) trial. CI Confidence interval, DAT high relapse activity before randomised treatment (see HRA) plus disease activity on treatment, EDSS Expanded Disability Status Scale, Gd+ gadolinium-enhancing, HRA high relapse activity before randomised treatment (≥ 2 relapses during the year prior to study entry), MRI magnetic resonance imaging; no evidence of disease activity = no relapses, no 3-month-confirmed EDSS worsening, no T1 Gd+ lesions, no active T2 lesions.
CLARITY Extension was a 2-year extension to CLARITY (n = 806) [15, 16]. The annualised relapse rate was 0.10 (95% confidence interval [CI] 0.06–0.13) for 186 patients who received cladribine tablets 3.5 mg/kg in both CLARITY and CLARITY Extension and 0.15 (95% CI 0.09–0.21) for 98 patients who received cladribine tablets 3.5 mg/kg in CLARITY and placebo in CLARITY Extension; 81.2 and 75.6% of patients, respectively, remained relapse-free over 4 years [15]. The relative risk (RR) reduction between these two cohorts (RR 0.65, 95% CI 0.39–1.08) did not reach statistical significance (p = 0.059). The authors concluded that “Cladribine tablets treatment for 2 years followed by 2 years’ placebo treatment produced durable clinical benefits similar to 4 years of cladribine treatment” [15]. The benefit-to-risk considerations therefore favour 2 years of treatment with cladribine tablets 3.5 mg/kg, rather than 4 years. This therapeutic response supports the hypothesis that therapy with cladribine tablets has the characteristics of an IRT, in that a course of treatment given over 2 years provided protection against relapse for the majority of patients for at least a further 2 years, without further treatment and despite recovery of median total lymphocyte counts to within the normal range.
There was some increase in lesion activity on the MRI scans in patients in CLARITY Extension who received cladribine tablets 3.5 mg/kg in the initial (2-year) phase and then received placebo in the extension phase [16]. This increased lesion activity was mainly in patients who entered the extension phase more than 43 weeks after the end of the randomised phase [16].
Cladribine tablets reduced the risk of conversion to clinically definite MS (CDMS) following a first demyelinating event in the randomised, placebo-controlled, 96-week Oral Cladribine in Early Multiple Sclerosis (ORACLE MS) trial (hazard ratio 0.33, 95% CI 0.21–0.51; p < 0.0001) [17]. It should be noted, however, that cladribine tablets do not have a therapeutic indication for use in people with clinically isolated syndrome (CIS) either in Europe [6] or in the USA [18]; indeed, its US labelling specifies that cladribine tablets are not recommended for the management of CIS [18]. However, the ORACLE trial was published in 2014; a substantial proportion of patients diagnosed with CIS at that time would now be considered to have CDMS according to the updated McDonald criteria for the diagnosis of MS [19].
Safety and Tolerability
Serious adverse events in the initial 2-year phase of the CLARITY study occurred in 8.4% of the cladribine tablets 3.5 mg/kg group and in 6.4% of the placebo group [10]. Infections presenting as a serious adverse event were uncommon in the cladribine tablets 3.5 mg/kg treatment arm (2.3% vs. 1.6% for placebo) and were mainly reactivation of herpes zoster (all restricted and dermatomal in nature) [10]. Three cases of tuberculosis (TB) occurred in patients receiving cladribine tablets 3.5 mg/kg for MS [5]. Screening for TB is now mandatory for all patients before initiating therapy with cladribine tablets 3.5 mg/kg according to European labelling [5]. Progressive multifocal leukoencephalopathy has not been observed at the time of writing this review in patients with MS taking cladribine tablets.
Limited data on malignancy are currently available for cladribine tablets 3.5 mg/kg [20]. The European Summary of Product Characteristics for cladribine tablets 3.5 mg/kg describes a higher incidence of malignancy versus placebo (0.29 vs. 0.15/100 patient-years); a similar statement is made in the US labelling using data from slightly longer safety observations [5, 18]. The incidence of malignancy appears to be no higher for cladribine tablets 3.5 mg/kg than for other DMDs [21], and there has been no evidence of clustering of any given type of malignancy [22].
Epidemiological comparisons with the international GLOBOCAN database reference population demonstrated a standardised incidence ratio (SIR; 95% CI) for any cancer (excluding non-melanoma skin cancer) of 0.97 (0.44–1.85) for a cohort who received oral monotherapy with cladribine tablets 3.5 mg/kg and 0.48 (0.14–1.53) for placebo [23]. Other cohorts provided similar results: the corresponding SIR for a placebo-controlled double-blind treatment cohort was 1.39 (0.59, 2.76) for cladribine tablets (no cases on placebo), while the SIRs for placebo and cladribine tablets for all exposed patients were 0.38 (0.11, 1.21) and 1.06 (0.70, 1.55) [23]. These results indicate there is no difference between the incidence of malignancies seen in patients who received cladribine compared with the general population. Overall, the apparent difference in cancer rates between cladribine tablets 3.5 mg/kg and placebo in the clinical trials database described above may have arisen from the unexpectedly low incidence of malignancy in the placebo control group of CLARITY [21].
Patients taking cladribine tablets should be advised to follow standard cancer screening guidelines.
Practical use of Cladribine Tablets 3.5 mg/kg in the Management of Multiple Sclerosis
Initiation—Who?
The (European) therapeutic indication is for highly active RRMS (Box 1) [5]. The drug is contraindicated in immunocompromised patients and in patients with active malignancy. Other contraindications and precautions relate mainly to populations that were not represented strongly in the clinical trials. Important contraindications relate to avoidance of pregnancy and breastfeeding during treatment, and these are summarised in Box 2 [5].
Lymphocyte counts must be normal before the first treatment course. To be eligible for treatment with cladribine tablets 3.5 mg/kg patients must be free from existing significant infections (screen especially for active TB or hepatitis and treat these successfully before initiating treatment, and also for latent or acute infections).
Vaccination for Herpes (varicella) zoster is recommended for patients previously unexposed to this virus. Treatment with cladribine tablets 3.5 mg/kg should be withheld for 4–6 weeks after the administration of a live or attenuated live vaccine, and other medications should not be taken within 3 h of taking this treatment.
Initiation—How?
As mentioned in the Introduction, “cladribine tablets 3.5 mg/kg” refers to a total cumulative dose of the drug given over 2 years. The tablet strength is 10 mg, and its labelling describes how many tablets a patient needs to take each week, according to the individual’s body weight [5, 18] Cladribine tablets are administered over 5 consecutive days initially (or 4 days for patients with body weight 40–50 kg), both at the start of treatment and again 1 month later. This treatment course is repeated 1 year later (subject to recovery of the lymphocyte count; see Box 3 [5]), with no requirement for further treatment (based on the CLARITY Extension study, see above). In summary, patients need to take one or two tablets daily, for 16 or 20 days in a 2-year period depending on their body weight.
The therapeutic indication for cladribine tablets is highly active MS (see Box 1 for the European indication; the US indication specifies that the treatment is “generally recommended for patients who have had an inadequate response to, or are unable to tolerate, an alternate drug indicated for the treatment of MS”). Accordingly, in practice, most patients who receive this treatment are likely to have responded sub-optimally to a previous DMD. Table 2 provides suggested guidance for switching from commonly used first- or second-line DMDs to cladribine tablets 3.5 mg/kg, such as that based on the European labelling for these DMDs and the experience of the authors [5, 24,25,26,27,28]. Immediate switching to cladribine tablets 3.5 mg/kg is consistent with its clinical indication [5] in cases where the previous DMD is eliminated quickly and where long-lasting suppression of lymphocyte counts is absent. Teriflunomide persists in the body for a period of months to years, and an accelerated washout procedure is available [26], while a 6-week washout of fingolimod should be sufficient to allow this agent to clear the body [28].
Monitoring Requirements
Little monitoring is required during treatment with cladribine tablets 3.5 mg/kg (Box 3). According to the European labelling, lymphocyte counts should be monitored 2 and 6 months after each treatment; where the lymphocyte count falls to < 500/mm3, this should be actively monitored until levels recover [5]. Labelling in the USA is similar, with a requirement to monitor lymphocyte counts to month 6 post-treatment where this is < 200/mm3 [18]. Pregnancy and infection status should be checked before the second course of cladribine tablets [5, 18].
Implications of Immune Reconstitution Therapy for the Care of Patients with Multiple Sclerosis
Patient preferences are increasingly at the core of clinical decision making [29]. The fear of many patients with MS of disease progression and disability can itself degrade quality of life [30, 31]. Most patients wish to receive individualised information about their prognosis [32] and may be prepared to make trade-offs between the greater expected efficacy and more challenging tolerability profiles of more effective DMDs. Preferences for oral versus injectable treatment, and the burden of monitoring required, may also influence a patient’s preference between different treatments [31, 33].
Planning for pregnancy is a further consideration (see Box 3). Cladribine tablets 3.5 mg/kg is contraindicated in this setting, and precautions to prevent pregnancy must be maintained for at least 6 months after treatment (of either partner), although its efficacy persists well beyond this period, with no additional treatment needed for several years in most patients after the treatment course in the second year.
Our aim in this review was to provide pragmatic recommendations for the administration of cladribine tablets as a relatively new therapeutic option for the management of MS. A detailed account of the relative efficacy and tolerability/safety of this agent is beyond the remit of this review, and the reader is referred to other sources for this information [34, 35]. One network meta-analysis (of 44 studies that evaluated 12 DMDs) compared cladribine tablets with other DMDs in people with MS [34]. The findings of this meta-analysis were that cladribine tablets were significantly more effective in reducing the annualised relapse rate than the “platform” or “first-line” therapies (interferons, glatiramer acetate, teriflunomide), they were similar to fingolimod and there was a non-significant trend towards slightly lower efficacy compared with alemtuzumab, natalizumab or ocrelizumab. Broadly similar results were obtained for measures of confirmed disability progression over different time periods. Cladribine tablets were more likely to result in NEDA than platform therapies, where data were available. There was no significant difference in the risk of adverse events between cladribine tablets and placebo, and most other DMDs, with a trend for more such events when patients were on alemtuzumab and interferon. It should be noted that the European Medicines Agency has recently restricted the indications for alemtuzumab due to concerns over rare, but serious, cardiovascular and autoimmune averse events, in particular [36].
Indeed, NEDA is emerging as a treat-to-target outcome for people with MS on a highly active DMD [37, 38]. The greater efficacy of cladribine tablets 3.5 mg/kg in preventing EDSS progression and increasing the likelihood of NEDA in patients with high disease activity at baseline in a post hoc analysis of the CLARITY study [13] (see Fig. 1) suggests that this agent is particularly suitable for patients at greater risk of future relapses and future disability. The CLARITY Extension study was not designed to determine the benefit of further courses of cladribine tablets in response to clinical or MRI events, so it has not been proven whether patients would benefit from additional course should disease recurrence occur. Additional courses of cladribine tablets were associated with an increased risk of certain safety events, particularly an increased risk of severe lymphopenia. These risks will need to be assessed against the potential benefits of additional courses, and new clinical data are needed to guide physicians in the long term. No formal therapeutic indication exists for continued treatment with cladribine tablets beyond 2 years at the present time.
Conclusions
Immune reconstitution therapy provides a radically different therapeutic approach to continuously administered maintenance therapies. Compared with DMDs that require continuous administration, pharmacologic IRT courses are short and intermittent, the potential for immunosuppression is transient and the period of suppression of MS disease activity long outlasts both the period of treatment and the duration of suppression of lymphocyte counts. Experience to date suggests that cladribine tablets 3.5 mg/kg acts like an IRT, as it demonstrates efficacy against disease activity for at least 2 years following the end of the second-year treatment. Therapy with cladribine tablets 3.5 mg/kg has a low potential for serious infections. An increased incidence of malignancy was seen for cladribine tablets versus placebo in the clinical trials. However, the placebo rate of malignancies was unexpectedly low, and there appeared to be no difference in malignancy rates for cladribine tablets compared to a matched reference population. The monitoring requirements associated with cladribine tablets are low, compared with most other high-efficacy DMTs, with minimal interference with patients’ daily lives.
References
Fyfe I. Multiple sclerosis: immune reconstitution effective for MS. Nat Rev Neurol. 2016;12:429.
Sorensen PS, Sellebjerg F. Pulsed immune reconstitution therapy in multiple sclerosis. Ther Adv Neurol Disord 2019;12:1–16.
Snowden JA, Sharrack B, Akil M, et al. Autologous haematopoietic stem cell transplantation (aHSCT) for severe resistant autoimmune and inflammatory diseases—a guide for the generalist. Clin Med (Lond). 2018;18:329–34.
Malmegrim KCR, Lima-Júnior JR, Arruda LCM, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: from mechanistic insights to biomarkers. Front Immunol. 2018;9:2602.
Mavenclad. European Summary of Product Characteristics. 2020. https://www.medicines.org.uk/emc/product/8435/smpc. Accessed Jan 2020.
Lemtrada®. European Summary of Product Characteristics. 2019. https://www.medicines.org.uk/emc/product/5409. Accessed Jan 2020.
Giovannoni G. Immune reconstitution in MS: How does this impact treatment decisions? Medscape Education. 2018. http://img.medscapestatic.com/images/892/112/892112_transcript.pdf. Accessed Jan 2020.
Karussis D, Petrou P. Immune reconstitution therapy (IRT) in multiple sclerosis: the rationale. Immunol Res. 2018;66:642–8.
Yatim KM, Lakkis FG. A brief journey through the immune system. Clin J Am Soc Nephrol. 2015;10:1274–81.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416–26.
Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post hoc and subgroup analysis. Lancet Neurol. 2011;10:329–37.
Rammohan K, Giovannoni G, Comi G, et al. Cladribine tablets for relapsing-remitting multiple sclerosis: efficacy across patient subgroups from the phase III CLARITY study. Mult Scler Relat Disord. 2012;1:49–54.
Giovannoni G, Soelberg Sorensen P, Cook S, et al. Efficacy of cladribine tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: a post hoc analysis of the CLARITY study. Mult Scler. 2019;25:819–27.
Ali S, Paracha N, Cook S, et al. Reduction in healthcare and societal resource utilization associated with cladribine tablets in patients with relapsing-remitting multiple sclerosis: analysis of economic data from the CLARITY Study. Clin Drug Investig. 2012;32:15–27.
Giovannoni G, Soelberg Sorensen P, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler. 2018;24:1594–604.
Comi G, Cook S, Rammohan K, et al. Long-term effects of cladribine tablets on MRI activity outcomes in patients with relapsing-remitting multiple sclerosis: the CLARITY Extension study. Ther Adv Neurol Disord. 2018;11:1756285617753365.
Leist TP, Comi G, Cree BA, et al. Effect of oral cladribine on time to conversion to clinically definite multiple sclerosis in patients with a first demyelinating event (ORACLE MS): a phase 3 randomised trial. Lancet Neurol. 2014;13:257–67.
Mavenclad® US Prescribing information. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022561s000lbl.pdf. Accessed Jan 2020.
van der Vuurst de Vries RM, Mescheriakova JY, Wong YYM, et al. Application of the 2017 revised McDonald Criteria for multiple sclerosis to patients with a typical clinically isolated syndrome. JAMA Neurol. 2018;75(11):1392–8.
Lebrun C, Rocher F. Cancer risk in patients with multiple sclerosis: potential impact of disease-modifying drugs. CNS Drugs. 2018;32:939–49.
Pakpoor J, Disanto G, Altmann DR, et al. No evidence for higher risk of cancer in patients with multiple sclerosis taking cladribine. Neurol Neuroimmunol Neuroinflamm. 2015;2:e158.
Galazka A, Nolting A, Cook S, et al. An analysis of malignancy risk in the clinical development programme of cladribine tablets in patients with relapsing multiple sclerosis (RMS). ECTRIMS Online Library. 2017; 199898:P1878. Abstract. https://onlinelibrary.ectrims-congress.eu/ectrims/2017/ACTRIMS-ECTRIMS2017/199898/vicky.john.an.analysis.of.malignancy.risk.in.the.clinical.development.html. Accessed Jan 2020.
European Medicines Agency. Assessment report. MAVENCLAD. International non-proprietary name: cladribine. Procedure No. EMEA/H/C/004230/0000. 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004230/WC500234563.pdf. Accessed Jan 2020.
Rebif®. European Summary of Product characteristics. 2020. https://www.medicines.org.uk/emc/product/8212/smpc. Accessed Jan 2020.
Copaxone®. European Summary of Product characteristics. 2019. https://www.medicines.org.uk/emc/product/183/smpc. Accessed Jan 2020.
Aubagio®. European Summary of Product characteristics. 2019. https://www.medicines.org.uk/emc/product/5244. Accessed Jan 2020.
Tecfidera®. European Summary of Product characteristics. 2020. https://www.medicines.org.uk/emc/product/5256/smpc. Accessed Jan 2020.
Gilenya®. European Summary of Product characteristics. 2020. https://www.medicines.org.uk/emc/product/4545. Accessed Jan 2020.
Cofield SS, Thomas N, Tyry T, Fox RJ, Salter A. Shared decision making and autonomy among us participants with multiple sclerosis in the NARCOMS Registry. Int J MS Care. 2017;1:303–12.
Nielsen J, Saliger J, Montag C, Markett S, Nöhring C, Karbe H. Facing the unknown: fear of progression could be a relevant psychological risk factor for depressive mood states among patients with multiple sclerosis. Psychother Psychosom. 2018;87:190–2.
Mansfield C, Thomas N, Gebben D, Lucas M, Hauber AB. Preferences for multiple sclerosis treatments: using a discrete-choice experiment to examine differences across subgroups of US patients. Int J MS Care. 2017;19:172–83.
Dennison L, Brown M, Kirby S, Galea I. Do people with multiple sclerosis want to know their prognosis? A UK nationwide study. PLoS One. 2018;13:e0193407.
Utz KS, Hoog J, Wentrup A, et al. Patient preferences for disease-modifying drugs in multiple sclerosis therapy: a choice-based conjoint analysis. Ther Adv Neurol Disord. 2014;7:263–75.
Siddiqui MK, Khurana IS, Budhia S, et al. Systematic literature review and network meta-analysis of cladribine tablets versus alternative disease-modifying treatments for relapsing-remitting multiple sclerosis. Curr Med Res Opin. 2018;34:1361–71.
Alroughani R, Inshasi JS, Deleu D, et al. An overview of high-efficacy drugs for multiple sclerosis: Gulf Region expert opinion. Neurol Ther. 2019;8:13–23.
European Medicines Agency. Lemtrada. Measures to minimise risk of serious side effects of multiple sclerosis medicine Lemtrada. 2019. https://www.ema.europa.eu/en/medicines/human/referrals/lemtrada. Accessed Jan 2020.
Marta M. No evidence of disease activity in people with multiple sclerosis. Eur J Neurol. 2019;26:1–2.
Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord. 2015;4:329–33.
Acknowledgements
Funding
Merck Serono Middle East FZ LTD funded this review and the Rapid Service Fee for this article.
Editorial Assistance
Amedical writer (Dr. Mike Gwilt, GT Communications) provided editorial assistance, funded by Merck Serono Middle East FZ LTD, an affiliate of Merck KGaA, Darmstadt, Germany. JKS and AS (employees of Merck N.V. S.A., an affiliate of Merck KGaA, Darmstadt, Germany) reviewed the manuscript for accuracy.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Ahmed El Boghdady is an employee of Merck Serono Middle East FZ LTD, an affiliate of Merck KGaA, Darmstadt, Germany. Mohamed AlJumah, Mona Marwan Alkhawajah, Shireen Qureshi, Ibtisam Al-Thubaiti, Omar Ayoub, Saeed A Bohlega, Areej Bushnag, Edward Cupler, Abdulkader Daif, Ahmed Hassan, Yaser Al Malik, Jameelah Saeedi, Fawzia Al-Shamrany, Eslam Shosha and Peter Rieckmann have acted as consultants to and speakers for Merck KGaA, Darmstadt, Germany.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to: https://doi.org/10.6084/m9.figshare.11617680.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
About this article
Cite this article
AlJumah, M., Alkhawajah, M.M., Qureshi, S. et al. Cladribine Tablets and Relapsing–Remitting Multiple Sclerosis: A Pragmatic, Narrative Review of What Physicians Need to Know. Neurol Ther 9, 11–23 (2020). https://doi.org/10.1007/s40120-020-00177-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-020-00177-5