Abstract
Introduction
Rutile TiO2 dioxide particles were modified with P2O5 treatment for improving its UV light fastness and the characteristics are studied using different techniques. The investigation aimed at improving the light fastness of the TiO2 particles by surface modification with P2O5 and optimizing its content for best performance. The coating of different inorganic oxides such as SiO2, Al2O3, P2O5, etc. were confirmed by atomic absorption spectroscopy. Microscopic evaluation was employed to evaluate the surface morphology of modified TiO2. The specific surface area was estimated using BET method. Effect of the surface modification with oxides on electrokinetic properties and zeta potential is appraised. Changes in the pigmentary properties on UV exposure are determined as a measure of light fastness.
Results
The light fastness of the modified samples is superior to commercial TiO2 pigments. The dispersion and pigmentary characteristics of the P2O5 modified samples have approached the commercial rutile TiO2 pigment standards. The reducing strength values indicate good dispersion characteristics of the P2O5 modified samples.
Conclusion
The light fastness of TiO2 particles can be improved considerably on imparting the coating of P2O5 along with Al2O3.
Similar content being viewed by others
Introduction
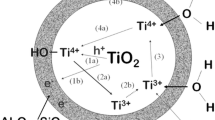
It is well known that the rutile form of titanium dioxide has been used as a white pigment in paints, plastics, paper industries, etc. for their excellent pigmentary properties. The titanium dioxide crystals are semi-conducting and show some intrinsic photocatalytic activity. On interaction of UV light with TiO2, free electrons and electron holes can be formed in the crystal lattice. In the presence of oxygen and water, formation of reactive free radicals is possible [1, 2]. Hydroperoxyl and hydroxyl radicals emerging from the crystal surface are formed on the surface of TiO2 crystal and react easily with neighboring organic molecules such as those from the binder medium and consequently, degradation of the medium can occur [1–4].
The interaction of UV light with TiO2 particle results in a particular reduction leading to the formation of Ti3+ centers. As Ti3+ is a violet colored species, some discoloration of the exposed area called graying might be observable. This is usually described in terms of the light fastness [2].
Commercially available TiO2 pigments have been normally surface modified with hydrated compounds of aluminum, silicon, and zirconium on the surface of pigment particles [5–8]. Many other literature focus on the optimum conditions with different coating process for excellent quality of pigmentary TiO2 particles [9–14]. Silica is generally added to improve the durability. Zirconia improves gloss and durability, whilst alumina aids dispersion and dispersion stability. But these treatments are not sufficient enough to minimize the migration of free radicals so as to accomplish good light fastness or otherwise these treatments do not satisfy the requirement of light fastness. For this reason, TiO2 pigments for end-use applications such as decorative papers requiring high light fastness need to be stabilized with some special surface treatment.
It is the purpose of this study to modify the TiO2 surface with an additional treatment of P2O5 to improve the light fastness. Compositional analysis and electrokinetic behavior were investigated to confirm the modification of the TiO2 surface with P2O5. The light fastness and other pigmentary properties were evaluated and compared against conventionally modified pigment grades.
Methods
Materials
All chemicals used in the experiments were of analytical reagent (AR) grade. Sodium silicate (26.5 % w/v SiO2, 10.6 % Na2O) was obtained from Aldrich. Aluminum trihydrate (65.2 w/w Al2O3) for preparing sodium aluminate (32.45 % w/v Al2O3) and phosphoric acid (88.4 %) were purchased from Nice Chemicals, India. Commercial TiO2 particles from the chloride process (with rutile crystals) without any surface coating were used in the experiments. The synthesized samples are labeled S-1 to S-12. Commercial rutile TiO2 samples with SiO2/Al2O3 and SiO2/Al2O3/ZrO2 modifications and an unmodified rutile TiO2 sample were taken for comparative evaluation and are labeled S-13, S-14, and S-15, respectively.
Apparatus and equipments
The chemical composition of the samples was determined using X-Ray Fluorescence spectrometer (S4 Pioneer, Bruker). The Al reduction technique (ASTM test method D1354-76) was employed to analyze the TiO2 content of the samples. Optical properties, such as L, (brightness) and b (color) of dry compressed TiO2 samples were determined using color spectrometer (CIELab, BYK-Gardener). The zeta potential versus pH curve of TiO2 samples was obtained through a zeta potential analyzer (Brookhaven Instruments, USA). The investigation on the morphology of modified TiO2 was carried out using scanning electro microscopy (Joel 6300F) and transmission electro microscopy (Joel 1200 EX2). The adsorption properties of selected samples were characterized by determination of their specific surface area using ASAP 2020 (Micrometrics Instruments Co.). Particle size distribution of samples was determined with the use of Microtrac X100 (Honeywell). The relative amount of light fastness or graying was measured by the following test [15]: a paste was prepared by rubbing in a glass muller 0.5 g of TiO2 pigment, 2.5 g of lead carbonate, and 0.75 ml of glycerol. The paste was spread between two glass slides and exposed under an UVA-340 lamp. The relative amount of graying or change in optical properties was determined at specific time intervals using the color spectrometer.
Reducing or Tinting strength, a measure of the light-scattering ability of the TiO2 pigment, was evaluated according to ASTM test method, D2745.
Experimental
A number of samples were prepared as part of the surface coating studies with varying concentrations of P2O5 and Al2O3, and SiO2 as part of optimization process of surface modification. The details of the surface modifications are listed in Table 1. A brief description of the preparation of sample No. 11 is described: 75 g of unmodified raw TiO2 pigment was dispersed in deionized (DI) water to prepare a suspension of 325 g/L concentration. The coating experiments were carried out in an insulated stirrer mounted cylindrical reactor in which the temperature and pH can be inspected on-line through a thermometer and a pH electrode, respectively. The temperature during precipitation studies was maintained at 60 °C. The suspension was thoroughly stirred for the best dispersed TiO2 suspension and required reagents were added drop wise to this suspension. In the first stage of preparation of sample-11, H3PO4 (0.35 ml) was added drop wise to the TiO2 suspension till the pH of the suspension lowered to 5.0. Thereafter, 1.3 ml H3PO4 was added simultaneously with 1.3 ml sodium aluminate to the suspension drop wise maintaining the pH at 5.0. The additions were performed over a period of 10 min. The suspension was allowed to cure for 15 min. Then 1.4 ml of sodium silicate was added to the suspension to precipitate the silica coating on pigment. This was followed by curing for 10 min. Subsequently the pH of the suspension was then raised to 7.5 with 1.3 ml sodium aluminate and maintained at 7.5 by simultaneous drop wise addition of 8.7 ml sodium aluminate and 2.4 ml H2SO4 (48 %) in the second stage. The additions were performed over 10 min. The suspension was kept under stirring for 15 min.
Same experimental procedure was followed to prepare other samples, the changes being the variation in the treatment reagent and its composition. The quantity of chemicals added for each treatment batches are also shown in Table 1.
Subsequent to the treatment, the suspensions were filtered and washed with DI water until the resistivity of the solution recorded at least 4000 Ω. The filter cake was then dried at 110 °C for 3 h and then powdered.
Results and discussion
Chemical composition
The chemical composition of the samples is presented in Table 2. The coating experiments have found to impart the Al2O3, SiO2, and P2O5 coating in the required range for all the samples prepared as a consequence of the treatments of sodium aluminate, sodium silicate and H3PO4, respectively. In the samples taken for comparative evaluation, S-13 has moderate coatings of Al2O3 (2.60 %) and SiO2 (2.20 %), while S-14 has been characterized with a heavier coating of Al2O3 (3.60 %). The presence of SiO2 and ZrO2 has been also identified in the S-14 sample. The unmodified sample contained a small proportion of Al2O3 resulted from the addition of AlCl3 to TiCl4 to aid rutilization during oxidation step in TiO2 manufacturing. The higher proportion of surface modifications for S-1 to S-12 against comparative grades S-13 and S-14 has attributed to the higher TiO2 content in the latter samples.
Dry properties
The dry L, and, b values of all the samples determined using color spectrometer are tabulated in Table 3. It is clear from the results that there are no considerable changes in the dry L and b values upon modifying the surface with P2O5. The slightly diminished L and increased b values for the dry S-11 and S-12 may be accounted to the surface coating of SiO2 imparted in these samples. The lack of any surface modification in S-15 resulted in higher brightness and lower color values.
Electrokinetics
Changes in electrokinetic potential or the zeta potential vs. pH behavior for all samples were studied as depicted in Fig. 1 (S-1 to S-6) and Fig. 2 (S-7 to S-12). The value of isoelectric point (IEP) of TiO2 is strongly related on the inorganic substance used for modification. For pure substance, the IEP corresponded to around pH 6 for TiO2 [14, 16], pH 2.5 for SiO2 [14], and pH 9 for Al2O3 [17]. The IEP at pH 5.9 for unmodified TiO2 (S-15) is in good agreement with the values reported in literature. IEP for S-2 (6.5) has shifted toward low pH values in comparison against S-1 (7.0) because of the SiO2 modification imparted to S-2. On the other hand, higher Al2O3 and lower SiO2 proportions accounts for IEP shifting toward basic region (7.5) in dependence on the surface charge for S-14, whereas the involvement of SiO2 in moderate amounts resulted in shift of the IEP toward lower pH values (6.8) in the case of S-13. The lower IEP values for S-10 and S-11 in comparison against S-14 confirms the surface modification of the former samples by P2O5.
Light fastness
The samples were tested for possible UV light sensitivity effects by performing photocatalytic tests under UV.Degree of darkness/grayness of the samples on exposure to UV light at different time intervals is reported in Table 4. The mass tone or ΔE values calculated in accordance with the CIELab formula as a function of time are depicted in Fig. 3 (S-1 to S-6) and Fig. 4 (S-7 to S-12). In non-light fastness TiO2 pigment, UV light stimulates the formation of Ti3+ ions which in turn impart a grayish color to TiO2. ΔE can be employed as a measure of the formation of Ti3+ centers under the influence of UV light. A greater numerical ΔE value for the exposed sample relative denoted higher degree of graying or lower light fastness. The trends in graying tendency shown by the four samples upon exposure to UV light are furnished below.
The marked graying tendencies of S-1, S-13, S-14, and S-15 samples were clearly visible with the naked eye. The change in brightness, ΔL and color Δb, and hence the ΔE values were least observed for S-7 to S-12 at all the time intervals. The ΔL, Δb and ΔE values increased with the SiO2 modification imparted on the surface as evidenced from the values of S-10 to S-12 suggesting decreased UV light fastness.
Among samples which exhibited improved UV light fastness, S-10 features the highest. The commercial samples, S-13 and S-14, unmodified TiO2 sample, S-15 and samples without and least P2O5 coating, S-1 and S-2, respectively, show pronounced graying.
Taking the results shown in Table 4 into account, it maybe concluded that the UV light sensitivity of TiO2 reduces to a considerable extent on the surface modification with P. The reagent formed by the co-precipitation of phosphate and alumina forms a coherent coating of aluminum phosphate on the TiO2 pigment particles when precipitated. The widely accepted mechanism of TiO2 photocatalytic oxidation is based on semiconductor band theory by which the electrons in the valence band of TiO2 are excited to form electron–hole pairs under UV radiation, and the photogenerated holes react with surface hydroxyl groups and water molecules to form hydroxyl radicals. Hydroxy radicals with strong oxidation ability can oxidize organic matrix to form CO2, H2O and other inorganic substances. Alternately the photo generated holes can react directly with organics and then induce further degradation. At the same time the photogenerated electrons can react directly with O2 adsorbed TiO2. The The P modification might have resulted in reduction of oxidizing species at the particle surface and hence resulted in pigment with enhanced light fastness compared to SiO2/Al2O3 or SiO2/Al2O2/ZrO2 encapsulation.
Reducing strength
The reducing strength of TiO2 pigment describes the light-scattering contribution relative to its light absorbing ability. The dispersion of TiO2 pigment is greatly determined by the reducing strength values of the pigment. The optical/pigmentary properties of TiO2 pigments for decorative paper applications are highly critical. A poorly dispersed TiO2 pigment will have low light-scattering efficiency and hence lower reducing strength and pigmentary properties mainly owing to flocculation or agglomeration in the media in which it is dispersed.
The values of reducing strength for the samples are tabulated in Table 5. S-14 with moderate SiO2/Al2O3 surface treatment was made the reference standard. The reducing strength values of all the P2O5-treated samples are found to be highly comparable or even better than the non-P2O5 coated ones, S-13 and S-14 which ensures that the modification of P2O5 on TiO2 particles has not made any negative impact on the light-scattering efficiency of the pigment. The unmodified sample exhibited poorer strength owing to lack of any surface coating.
Particle size
The particle size distribution curves, taking into account the particle volume, for S-11, S-13, and S-14 are depicted in Fig. 5. The distribution of P2O5-modified as well the commercial TiO2 are found to fall within the range of 0.1 to 1.0 μm indicating narrow particle size distributions typical of pigmentary quality TiO2. The comparisons of the particle size distribution obtained for these samples revealed that the distribution of commercial TiO2, S-13, and S-14, exhibited similar pattern while the modifications in S-11 brings about a slighter shift in the pattern toward higher particle diameter region due to the additional coating of alumina and phosphate imparted on TiO2 surface. This is also ascertained from the comparatively higher average particle size (volume) values of 0.392 μm for S-11, 0.348 μm for S-13 and 0.339 μm for S-14. The higher degree of surface modification for P2O5-modified sample and greater tendency for particle agglomeration can be attributed for the higher average particle diameter and shift in the curve for alumina and phosphate modified S-11.
Morphology
The morphology of S-11 sample, which exhibited very good light fastness and highest reducing strength, observed through SEM and TEM are shown in Fig. 6a, b. The spherical particle shape and presence of primary aggregates was manifested from the SEM image where particle size of about 0.3 µm is estimated which is characteristic of pigment grade titanium dioxide. The TEM image provides an excellent view of the shell covering of crystalline core of titanium dioxide with inorganic oxides while leaving intact the core.
Surface area
The determination of absorption capacities of S-11, S-13, and S-14 area shows that the amount of nitrogen adsorbed by the modified sample to be higher. S-11 manifested the highest surface area which amounted to 27 m2/g reflecting higher surface modifications with Al2O3 and P2O5. A lower specific surface area of 19 m2/g was shown by S-13 and the lowest value of BET specific surface area was noted for S-14 (17 m2/g). Modifications with Al2O3 and P2O5 have found to significantly increase the specific surface area.
The mean diameter (S p) and volume of pores (V p) values are found to be higher for the P2O5-moidifed TiO2 sample than the commercial TiO2 samples (Table 6). Effect of modification might reflect in increasing amounts of inorganic oxides used for surface modification of TiO2. The surface area has increased due to the presence of higher number of active centers on the surface.
The mechanism behind the enhanced UV light fastness of modified TiO2 by the proposed method is explained as follows. A layer of aluminum-phosphorous compounds, and in a mixture with hydrous aluminum oxide is deposited on the surface of the TiO2 particles. The reagent formed by the co-precipitation of phosphate and alumina forms a coherent coating of aluminum phosphate on the TiO2 pigment particles when precipitated. Short –O–P–O–Al–O–P– chains are formed in solution during reaction between aluminum compounds and the phosphoric acid. When the pH is raised these short chains condense into longer chains and networks which have high affinity for the pigment surface and cover it completely. Possible deduction is that the modification reduces the generation of oxidizing species, according to the photocatalytic mechanism, at the particle surface either by modifying the intrinsic photocatalytic activity properties of titanium dioxide, thus improving the light fastness. Meanwhile the outer Al2O3 coating will impart the pigmentary qualities such as dispersion, gloss, etc. which are characteristic of micron-sized TiO2 pigment.
Conclusion
The TiO2 modified by the proposed method, which exhibited superior light fastness, was characterized by various analytical techniques. The experimental results reveal that the absolute value of zeta potential of the samples subjected to H3PO4 treatment has shifted to the acidic range, thus confirming the TiO2 pigment surface to be significantly modified with P2O5. The presence of surface modification with inorganic oxides is evaluated by compositional analysis. The surface modification was supported by the compositional, particle size, surface area, and SEM/TEM analyses. The modified TiO2 sample has demonstrated presence of spherical particles and its particle size distribution pattern indicate that surface modification with inorganic oxides such as Al2O3 and P2O5 exerts some effect on character and increase the diameter of the particles. The absorptive capacity is increased by this modification and the BET surface area has increased due to the presence of higher number of active centers on the surface. The generation of oxidizing species at the particle surface is decreased by the presence of P modification and hence resulting in enhanced UV light fastness. Optimization studies revealed that TiO2 modified with around 2.5 % P2O5 and 5.5 % Al2O3 yielded superior light fastness. The aluminum phosphate coating on the TiO2 surface has been found to improve the light fastness of TiO2 in comparison against commercial pigments modified with SiO2/Al2O3 and SiO2/Al2O2/ZrO2. The high light-scattering efficiency evidenced for the synthesized TiO2 particles modified with P2O5 indicates good dispersion and pigmentary characteristics of the same. The results of the analysis of optical properties of the dry compressed pigment samples support the same.
References
Diebold MP (1995) The causes and prevention of titanium dioxide induced photo- degradation of paints. Part I: theoretical considerations and durability. Surf Coat Int 6:250–256
Woditsch P, Westerhaus A (1993) Industrial inorganic pigments, 3rd edn. VCH Weinheim, New York
Li X, Cubbage JW, Tetzlaff TA, Jenks WS (1999) Photocatalytic degradation of 4-chlorophenol. 1. The hydroquinone pathway. J Org Chem 64:8509–8524
Li X, Cubbage JW, Jenks WS (1999) Photocatalytic degradation of 4-chlorophenol. 2. The 4-chlorocatechol pathway. J Org Chem 64:8525–8536
Decolibus RL TiO2 pigment coated with porous alumina/silica and dense silica. US Patent No. 3928057 1975 assigned to E. I. Du Pont de Nemours & Company, Wilmington, DE
Thomas DC Process of treating titanium dioxide pigments. US Patent No. 3876442 1975 assigned to Kerr-McGee Chemical Co., Oklahoma City, OK
Jacobson HW Alumina coated TiO2. US Patent No. 4416699 1983 assigned to
Tuomo L Process of coating titanium dioxide pigments. US Patent No. 5165995 1992 assigned to Kemira Oy, Helsinki, FI
Gesenhues U (1994) Coprecipitation of hydrous alumina and silica with TiO2 pigment as substrate. J Colloid Interface Sci 168:428–436
Werner AJ Titanium dioxide pigment coated with silica and alumina. US Patent No. 3437502 1969 assigned to E. I. Du Pont de Nemours & Company, Wilmington, DE
Howard PB Treatment of pigment. US Patent. 4052222 1977 assigned to Tioxide Group Limited, Cleveland, EN
Johansson LS, Tuomo L (1991) Surface characterization of coated powders: Al2o3-SiO2 coated TiO2. Surf Interface Anal 17:230–236
Bruni M, Visca M, Garbassi F, MelloCeresa E (1985) Precipitation of aluminosilicates on the surface of titanium dioxide. Ind Eng Chem Prod Res Dev 24:579–586
Lin YL, Wang TJ, Jin Y (2002) Surface characteristics of hydrous silica-coated TiO2 particles. Powder Technol 123:194–198
Toivo E, Virpi L Process of producing light proof titanium dioxide pigment. US Patent No. 3981737 1976 assigned to Kemira Oy, SF
Yang HG, Li CZ, Fang HCG (2001) Rheological behavior of titanium dioxide suspensions. J Colloid Interface Sci 236:96–103
Stefanska KS, Krysztafkiewicz A, Jesionowski T (2008) Effect of inorganic oxides treatment on the titanium dioxide surface properties. Physicochem Probl Min Proc 42:141–152
Acknowledgments
The authors sincerely thank, Mr. C.J George (Executive Director, KMML, India) and Mr. Suresh Kumar (Technical Advisor, KMML, India) for providing the opportunity and support.
Conflict of interest
The authors declare that there is no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
George, J., Gireesh, V.S., Ninan, G. et al. Modification of TiO2 surface for improved light fastness. Int J Ind Chem 6, 133–141 (2015). https://doi.org/10.1007/s40090-015-0044-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-015-0044-x