Abstract
The present investigation deals with the facile synthesis and characterization of chitosan (CTS)-based zinc oxide (ZnO) nanoparticles (NPs) and their antimicrobial activities against pathogenic microorganisms. ZnO–CTS NPs were synthesized through two different methods: nano spray drying and precipitation, using various organic compounds (citric acid, glycerol, starch and whey powder) as stabilizers. Both the synthesis methods were simple and were devoid of any chemical usage. The detailed characterization of the NPs was carried out using UV–Vis spectroscopy, dynamic light scattering particle size analysis, zeta potential measurements and scanning electron microscopy, which confirmed the fabrication of NPs with different shapes and sizes. Antimicrobial assay of synthesized ZnO–CTS NPs was carried out against different pathogenic microbial strains (Candida albicans, Micrococcus luteus and Staphylococcus aureus). The significant (p < 0.05) inhibition of growth was observed for both M. luteus and S. aureus with ZnO–CTS NPs (with a concentration ranging from 0.625 to 0.156 mg/ml) as compared to control treatment. ZnO–CTS NPs also showed significant biofilm inhibition activity (p < 0.05) against M. luteus and S. aureus. The study demonstrated the potential of ZnO–CTS NPs as antimicrobial and antibiofilm agents.
Similar content being viewed by others
Background
Metal oxide nanoparticles (NPs), such as zinc oxide (ZnO), have been found to exhibit interesting properties, such as large surface-to-volume ratio, high surface reaction activity, high catalytic efficiency, and strong adsorption ability [1–3], that make them potential candidates for various applications. ZnO is an inorganic material with several advantages, such as wide band gap (3.34 eV), semiconductor with a high exciton binding energy (60 meV), high isoelectric point (9.5), and fast electron transfer kinetics [4, 5]. All these features suggest the feasibility for synthesis of special ZnO nanostructures. Nano ZnO can be synthesized in many forms: rods, wires, whiskers, belts, bipods, tetrapods, tubes, flowers, propellers, bridges, and cages [6–8]. Nanostructured ZnO has great potential for many practical applications, such as dye sensitized solar cells, piezo electric transducers, UV-light emitters, chemical and gas sensors and transparent conductive coating [9]. It also exhibits intense ultraviolet absorption and can potentially be utilized as UV-shielding materials and antibacterial agents [10]. ZnO–NPs have been prepared by techniques, such as the sol–gel method [11, 12], precipitation [13], hydrothermal synthesis [14], and spray pyrolysis [15].
Recently, chitosan (CTS) hybrid materials including conducting polymers, metal NPs and oxide agents have been developed with excellent properties of individual components and outstanding simultaneous synergistic effects [16]. Currently, the research on the combination of CTS and metal oxide has focused on titanium dioxide (TiO2), as it possesses excellent photo catalytic performance and is stable in acidic and alkaline solvents. In parallel with TiO2, ZnO also has similar band gap and antibacterial activity [16]. The cationic nature of CTS makes it possible to adhere to the negatively charged surface, such as bacterial cell membranes. CTS is soluble in dilute acidic solutions and interacts with polyanions to form complexes and gels. CTS is innocuous and possesses antibacterial and antifungal properties [17, 18]. CTS has tremendous ability to form metal complexes with Zn metal [19]. Owing to the presence of amine and hydroxyl groups on CTS, currently ZnO–CTS complex attracted great interest for its potential use as UV protector and medicament.
However, due to the extremely high reactivity, the initially formed ZnO NPs tend to react rapidly with the surrounding media or agglomerate, resulting in the formation of much larger (µm to mm scale) particles or flocs. In the process, they rapidly lose their bioactivity. In order to prepare more stable and active ZnO NPs, studies have been carried out either by the fabrication on the surface of NPs by organo-alkoxysilane [20] or by grafted polymers [21]. However, only few studies have been carried out on the surface modification of NPs to create a stable dispersion in aqueous medium. Various organic compounds, such as citric acid (CA), glycerol, starch and whey powder (contains lactose moiety), among others can be used as capping agents. These water-soluble organic compounds serve as a stabilizer and dispersant that prevents the resultant NPs from agglomeration, thereby prolonging their reactivity and maintaining the physical integrity. Moreover, these organic compounds are safe and innocuous.
The present work was carried out with following objectives: (1) facile synthesis and characterization of ZnO–CTS NPs with different stabilizers using nano spray drying and precipitation method. Various organic compounds, such as CA, glycerol, starch and whey powder, were used as capping agents to stabilize the NPs formulation and; (2) application of ZnO–CTS NPs as antimicrobial and antibiofilm forming agents against pathogenic microbial strains, e.g., Candida albicans, Micrococcus luteus and Staphylococcus aureus. These strains were selected due to their widespread occurrence and resulting health hazards. C. albicans is a causal agent of opportunistic oral and genital infections in humans [22]. Systemic fungal infections (fungemias) by C. albicans have emerged as major causes of morbidity and mortality in immuno-compromised patients. Candida albicans forms biofilms on the surface of implantable medical devices, as a consequence hospital-acquired infections by C. albicans have become a cause of major health concerns. Micrococcus luteus is a Gram-positive, spherical, saprotrophic bacterium that belongs to the family Micrococcaceae. Micrococcus luteus is found in soil, dust, water and air, and as part of the normal flora of the mammalian skin. It also colonizes the human mouth, mucosae, oropharynx and upper respiratory tract. Micrococcus luteus shows higher potential for biofilm formation. Staphylococcus aureus is a facultative anaerobic Gram-positive coccal pathogenic bacterium belonging to Staphylococcaceae family. Staphylococcus aureus is the prevalent cause of food intoxication and can cause a wide range of illnesses, from minor skin infections to life-threatening diseases, such as pneumonia, meningitis, osteomyelitis, endocarditis, bacteremia and sepsis. It is one of the five most common causes of nosocomial infections and is often the cause of postsurgical wound infections [23].
Materials and methods
Chemicals
Yeast Peptone Glucose (YPG) medium, tryptic soya broth and agar were purchased from Fischer scientific. Zinc oxide (ZnO) and CA were supplied by Sigma-Aldrich (Ontario, Canada). CTS (molecular weight- 600–800 kDa and degree of deacetylation (DD) >90 % and viscosity 200–500 mPa s) and starch (soluble, ACS grade) were purchased from Fisher Scientific (Ontario, Canada). Whey powder (whey permeate) was purchased from Agropur Cooperative, Québec, Canada. Milli-Q water was prepared in the laboratory using a Milli-Q/Milli-RO Milli pore system (Milford, MA, USA). Glycerol was purchased from Labmat Inc. Québec, Canada. The composition or properties of whey powder were as follows: carbohydrates (mainly as lactose) >82.0 %; protein ≤3.5 %; fat <0.3 %; ash content 8.0–9.0 %; moisture <5.0 %; pH (10 % solution) 6.1 and titrable acidity of 0.12 %.
Preparation of ZnO–CTS NPs
Nano spray drying method
The synthesis of ZnO–CTS NPs was carried out using nano spray dryer (B-90 BUCHI, Switzerland). NPs were prepared by dissolving 0.75 g of ZnO powder in 100 ml of 1 % (v/v) acetic acid. This led to the dissociation of ZnO to zinc cations. 1 g of CTS was added to this solution. After fully dissolving the CTS, different organic compounds [1 % (w/v) CA; 0.5 % (w/v) whey powder; 0.5 % (w/v) soluble starch and 0.5 % (w/v) glycerol] were added as stabilizers in various treatments. For comparison, control of ZnO NPs and ZnO–CTS NPs was prepared in the absence of stabilizer following the similar procedure. The process conditions were as follows: spray speed was 100 %; inlet and head temperature was set at 120 ± 1 °C. The pump speed was adjusted on standard mode of 1 and gas pressure was adjusted at 40–45 hPa which corresponds to a gas flow of around 150–160 l/min. The NPs formed were manually collected using particle collection paper.
Precipitation method
The similar procedure as nano spray dryer was followed and after making the solution containing ZnO–CTS in the absence or presence of stabilizer, the mixture was sonicated for 30 min. With magnetic stirring, 1 M NaOH was added until the solution attained pH 10. The samples were heated in water bath at 80 ± 1 °C for about 3 h followed by frequent washing with distilled water and lyophilization using a freeze dryer (ScanVac Cool Safe, LaboGene, Lynge, Denmark).
Analytical measurement
Viscosity of different samples was measured using a rotational viscometer Brookefield DVII PRO + (Brookfield Engineering Laboratories, Inc., Stoughton, MA, USA) equipped with Rheocalc32 software. The viscosity data acquisition and analysis were carried out using Rheocalc V2.6 software (B.E.A.V.I.S.––Brookfield Engineering Advanced Viscometer Instruction Set). All measurements were performed at 25 ± 1 °C, 36.69 s−1 shear rate and viscosity was referred to as “apparent viscosity”. pH of the different metal oxide solutions was measured with the pH meter equipped with glass electrode.
Characterization of NPs
UV–Vis spectrum
Absorption spectra of the NPs in aqueous suspension were recorded using a UV–Vis spectrophotometer (UV 0811 M136, Varian, Australia) at 200–600 nm. The optical path length of the cuvettes used in all the experiments was 1 cm. The absorption spectra were recorded at room temperature.
Size, zeta potential measurements and scanning electron microscope (SEM)
The mean size, size distribution and zeta potential measurements of the ZnO–CTS NPs were performed by Nano Zetasizer (Nano series, Malvern instruments Ltd., Worcestershire, UK) equipped with multipurpose titrator (MPT-2). The morphology of the synthesized ZnO NPs was examined by a scanning electron microscope (Model: Carl Zeiss EVO® 50 smart SEM) with a magnification of 10.0 K X, working distance (WD) 20 mm, secondary electrode 1 (SE 1) detector and EHT 10 kV. Samples were coated with gold before SEM testing.
Assessment of in vitro antimicrobial and antibiofilm activity of ZnO–CTS NPs
In this study the fungal strain, Candida albicans CCRI 10,345, and bacterial strains, Micrococcus luteus CCRI 16,025 and Staphylococcus aureus CCRI 20,630, were used to assess antimicrobial and antibiofilm activity of ZnO–CTS NPs formulations. The microbial strains were cultured for 48 h using Yeast Peptone Glucose (YPG) medium agar plates (C. albicans) and tryptic soya agar plates (M. Luteus and S. aureus).
The qualitative antimicrobial assay
The in vitro qualitative screening of the different ZnO–CTS NPs was performed using agar diffusion method. The filter paper discs (5 mm diameter) were dipped in each filter sterilized tested sample (NPs concentration of 5 mg/ml) and were placed in Petri dishes with yeast extract, peptone and glucose (YPG) (for fungi) and tryptic soya agar (for bacteria) medium previously seeded with the bacterial inocula. The inoculated plates were incubated for 24 h at 30 ± 1 °C. Antimicrobial activity was assessed by measuring the inhibition zone diameter (mm). Based on the qualitative assay results, only the bacterial strains susceptible to tested samples have been further tested in the quantitative assay using liquid medium.
The quantitative antimicrobial assay of the minimal inhibitory concentration
The quantitative assay was performed in liquid medium with twofold serial dilutions of NPs solution (ranging between 5.0 and 0.156 mg/ml) using susceptible strains assessed through qualitative assays. The assay was performed in 96-well plates using tryptic soy broth medium. In brief, 200 µl volume of nutrient medium was poured in each well and inoculated with 20 µl inocula of 48 h grown cultures.
The microplates were incubated at 30 ± 1 °C for 24 h. The antimicrobial activity was quantified by measuring the optical density at 600 nm and minimum inhibitory concentration (MICs) was read as the last concentration of the tested compounds, which inhibited the microbial growth.
Antibiofilm activity
The antibiofilm activity was performed using the micro titer method. To do so, the microbial strains were grown in the presence of twofold serial dilution of NPs (ranging between 5.0 and 0.156 mg/ml) in liquid nutrient medium distributed in 96-well plates. In brief, 200 µl medium with known concentrations of tested sample was inoculated with 20 µl bacterial suspension and incubated for 24 h at 30 ± 1 °C for bacterial strains. At the end of the incubation time, the plastic wells were emptied, washed three times with phosphate buffer saline (PBS), fixed with cold methanol, and stained with 1 % violet crystal solution for 30 min. The biofilm formed on plastic wells was resuspended in 30 % acetic acid. The intensity of the colored suspension was assessed by measuring the absorbance at 492 nm [24]. The last concentration of the tested compound that inhibited the development of microbial biofilm on the plastic wells was considered as the MICs of the biofilm development and was also expressed in mg/ml [25].
Statistical analysis
The quantitative antimicrobial assay and biofilm inhibition assay were performed in triplicates and values are given as average ± SD. The data were statistically analyzed using the multiple analysis of variance (ANOVA) by SPSS (16.0 version). To determine the significance of difference between different treatments and concentrations of ZnO–CTS NPs for antimicrobial and biofilm inhibition of M. luteus and S. aureus, the test was performed at the level of p < 0.05.
Results and discussion
ZnO–CTS-based NPs synthesis and characterization
UV–Vis spectra
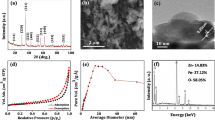
The UV–Vis spectra obtained for different ZnO–CTS NPs prepared through nano spray drying and precipitation method are given in Fig. 1a, b. The results indicated the formation of ZnO–CTS NPs. The synthesis of ZnO–CTS NPs is clearly evident, as the excitation peak was observed due to ZnO–CTS NPs at 273 nm, which lies much below the band-gap wavelength of 388 nm (E g = 3.2 eV) of ZnO. It was observed that absorption peaks of ZnO NPs prepared in the presence of stabilizers were prominent as compared to ZnO–CTS NPs without stabilizers. The absorption peak for a suspension of NPs is broader and determined by the distribution of particle size. In the smaller wavelength range, particles with smaller size contribute more and at the region of absorbance maximum, all particles contribute to the absorbance [26]. Therefore, the average particle size of ZnO–CTS NPs observed in the presence of stabilizers was lower as compared to the ZnO NPs prepared in the absence of stabilizers.
Size and zeta potential
The mean size and size distribution of various ZnO–CTS NPs with different stabilizers and synthesized through different methods are provided in Table 1. The mean size of the ZnO–CTS NPs synthesized through nano spray dying method ranged between 93.2 and 403 nm. The mean size of ZnO–CTS–glycerol NPs (402.5 nm) was even larger than the control (ZnO–NPs, 215 nm). The larger size of NPs may be due to the aggregation of NPs which affects their mean size. The concrete reason for aggregation is not clearly understood. The size distribution is also highly variable with peak 1 (1,196 nm with intensity of nearly 85 %) and peak 2 (9.29 nm with intensity of 14.4 %). This indicates that the concentration of ZnO, CTS and glycerol needs to be optimized to get discrete, uniform and dispersed NPs. In all other treatments, there is not much variation between the mean size and size distribution.
The mean size of ZnO–CTS NPs with different stabilizers and synthesized through precipitation lies between 99.3 and 603 nm. The ZnO–CTS followed by ZnO–CTS–starch exhibits the large size NPs with diameter of 359 and 285 nm, respectively. The ZnO–CTS NPs formulation showed some cross linking or aggregation which impacts their mean size and size distribution. There was not much variation between the mean size and size distribution in other treatments. The NPs obtained in the present study can be further tailored with specific size, uniformity and highly dispersed nature befitted for specific applications through optimization of different variables, such as concentration of metal, CTS and stabilizers.
The zeta potential values of −7.54, −12.9, −1.90, −24.7 and −35.5 mV were calculated for ZnO, ZnO–CTS, ZnO–CTS–glycerol, ZnO–CTS–starch and ZnO–CTS–whey NPs synthesized through nano spray drying method, respectively. Similarly, NPs fabricated via precipitation method resulted in zeta potential values of −17.9, −2.68, −37.3, 1.5, −12.8 and −11.0 mV for ZnO, ZnO–CTS, ZnO–CTS–CA, ZnO–CTS–glycerol, ZnO–CTS–starch and ZnO–CTS–whey NPs, respectively. Higher zeta potential value observed for NPs was prepared in the presence of different stabilizer except for ZnO–CTS–glycerol NPs in both methods. Higher zeta potential values can be due to the higher intensity of smaller size NPs in the medium with different stabilizers. Generally, the particles come closer and flocculation (aggregation of particles to form large size particle) takes place at low zeta potential and vice versa. Based on DLVO (Derjaguin, Landau, Verwey, and Overbeek) theory, a higher (negative) value of zeta potential is expected to result in larger electrostatic repulsion within the particles, leading to a higher shear sensitivity between particles and, consequently, smaller particle sizes.
SEM of NPs formed through nano spray drying and precipitation method
The scanning electron micrographs for ZnO–CTS NPs prepared by nano spray drying and precipitation method using different stabilizers are given in Figs. 2a–f,3a–f. As evident from the micrographs, the NPs with different size and shapes (circular, elliptical and nano rods) were synthesized depending on the different treatments and methods.
As evident from the results, ZnO NPs synthesized by the nano spray drying method were agglomerated and rod type. It can be inferred that in the absence of CTS and stabilizer, the resultant ZnO NPs do not appear as discrete nanoscale particles and form much bulk dendritic floc-like structures with varying density. This type of aggregation was mainly due to the high surface energy of ZnO NPs [21] leading to formation of bulk structures with lower reactivity. On the contrary, ZnO–CTS NPs were observed to be more distinct and uniform as compared to ZnO NPs. The ZnO–CTS–CA NPs were not synthesized using nano spray drying process. The reason can be the formation of viscous solution (Viscosity 4.50 mPa s) indicative of some polymer formation due to the presence of various functional groups on both CA and CTS resulting in some interaction between them. The nozzle of the nano spray dryer was blocked with viscous solution of ZnO–CTS–CA which resulted in the formation of thin film on the nozzle exit. ZnO–CTS–glycerol NPs were also found to be less discrete and elliptical type. ZnO–CTS–starch and ZnO–CTS–whey stabilized NPs were observed to be circular in shape. ZnO–CTS–starch NPs were porous structures whereas ZnO–CTS–whey NPs were found to be more discrete and well dispersed as compared to ZnO–CTS–starch NPs. The presence of whey contributed to the steric hindrance between ZnO–CTS NPs and prevented their aggregation and thus maintained the high surface area and reactivity of the particle.
In comparison, ZnO NPs formed by precipitation technique (Fig. 3a–f) were discrete and less variable as compared to ZnO NPs formed through nano spray drying method. ZnO–CTS NPs formed by precipitation were circular but agglomerated. ZnO–CTS–CA NPs were found to be uniform, distinct and very well dispersed. ZnO–CTS–glycerol NPs appeared to be bulk dendritic floc-like structures with non-discrete appearance. On the other hand, ZnO–CTS–starch and ZnO–CTS–whey NPs were found to possess rod-like aggregated structures. Studies demonstrated the multifunctional role of citrate ions in the NPs synthesis process, such as acting as a reducing agent, a stabilizer, and a complex agent [27, 28]. Ji et al. [27] suggested that the citrate ions act as reducing agents, stabilizers, and pH mediators in the synthesis of gold NPs. Various studies have shown that the growth of silver nanocrystals in solution is sensitive to the presence of CA or sodium citrate. However, the precise role of citrate ions still remains dubious. For instance, studies demonstrated that the citrate ions act as stabilizers in an efficient synthesis method for generating mono-dispersed silver nanoprisms through illumination of visible light [29, 30].
Applications of ZnO–CTS NPs
Antimicrobial activity
Qualitative antimicrobial assay
The results of qualitative antimicrobial assay using ZnO–CTS NPs are provided in Table 2. As evident from the inhibition zone diameter measurements, it can be inferred that the ZnO–CTS NPs were more effective towards M. luteus and S. aureus as compared to fungal strain C. albicans. Based on the qualitative antimicrobial assay results, M. luteus and S. aureus were further selected for quantitative assay and biofilm inhibiting activity testing.
Quantitative antimicrobial assay
The antimicrobial activity of ZnO–CTS NPs against M. luteus and S. aureus is shown in Tables 3 and 4. As evident from decrease in the OD (600 nm), the significant (p < 0.05) inhibition of growth was observed for M. luteus and S. aureus using ZnO–CTS NPs prepared through both nano spray drying and precipitation method. ZnO–CTS NPS showed maximum inhibition at lower concentrations (0.625–0.156 mg/ml) (p < 0.05) against both M. luteus and S. aureus. However, increasing the concentration of ZnO–CTS NPS above 0.625 mg/ml, lower growth inhibition was observed.
ZnO–CTS NPs prepared by nano spray drying method showed 51.6–73.0 % growth inhibition of M. luteus at concentration of 0.156 mg/ml. Similarly, ZnO–CTS NPs prepared by precipitation method showed 56.8–73.7 % growth inhibition of M. luteus at concentration of 0.156 mg/ml. Significant (p < 0.05) growth inhibition of S. aureus was observed with ZnO–CTS NPs concentration of 0.156 mg/ml as compared to higher concentrations. ZnO–CTS NPs prepared by nano spray drying and precipitation method showed growth inhibition of 62.0–73.4 and 65.2–82.0 %, respectively, for S. aureus at concentration of 0.156 mg/ml. ZnO–CTS CA NPs prepared by precipitation method showed promising results with nearly 82 % growth inhibition at concentration of 0.156 mg/ml. No significant (p > 0.05) differences in M. luteus and S. aureus growth inhibition were observed between ZnO–CTS NPs prepared by two different methods, namely nano spray drying and precipitation method.
Unlike ZnO–CTS NPs, CTS and ZnO powder exerted their growth inhibition effect at higher concentration of 5.0 mg/ml. Significant (p < 0.05) inhibition of growth was observed for both M. luteus and S. aureus at higher dose of 5.0 mg/ml for CTS and ZnO powder. However, decreasing the concentration of CTS and ZnO powder to 0.625 mg/ml and below showed non-significant (p > 0.05) inhibition. The growth inhibition showed by bulk ZnO and CTS powder at higher concentrations of 5 mg/ml was found to be significantly lower than ZnO–CTS NPs.
An important aspect of the use of ZnO as an antibacterial agent is its non-toxic nature towards human cells which is also the prerequisite for any antimicrobial agent [31]. ZnO is currently being investigated as an antibacterial agent in both micro-scale and nanoscale formulations. Various studies have demonstrated that ZnO NPs showed antibacterial activity [32–39]. As evident from this study, the antimicrobial activities displayed by ZnO NPs were higher as compared to micro particles [39]. The exact mechanisms of the antibacterial action have not yet been clearly elucidated. However, research advocated various mechanisms, such as the role of reactive oxygen species (ROS) generated on the surface of the particles [40], zinc ion release [36], membrane dysfunction [36, 37], and NPs internalization [33]. In the present study, the growth inhibition of both M. luteus and S. aureus with ZnO–CTS NPs can be attributed to the above mentioned mechanisms exerted by the ZnO NPs.
Antibiofilm activity
The biofilm inhibition activity of ZnO–CTS NPs against M. luteus and S. aureus is shown in Tables 5 and 6. ZnO–CTS NPs prepared through nano spray dying and precipitation methods showed significant (p < 0.05) biofilm inhibition activity against M. luteus and S. aureus at a lower concentration ranging from 0.625 to 0.156 mg/ml.
For M. luteus at NPs concentration of 0.156 mg/ml, ZnO–CTS NPs prepared by nano spray drying and precipitation method showed biofilm inhibition of 59.4–66.4 and 66.4–77.0 %, respectively. No significant difference (p > 0.05) was observed between different ZnO–CTS NPs prepared by nano spray drying method for biofilm inhibition at NPs concentration ranging from 0.625 to 0.156 mg/ml. However, significant difference was observed between ZnO NPs and ZnO–CTS NPs prepared by precipitation method on biofilm inhibition for M. luteus. Likewise, for S. aureus at NPs concentration of 0.156 mg/ml, ZnO–CTS NPs prepared by nano spray drying and precipitation method showed biofilm inhibition of 69.0–84.1 and 67.5–80.2 % respectively. No significant difference (p > 0.05) was observed on biofilm inhibition between different ZnO–CTS NPs prepared by nano spray drying and precipitation method at all NPs concentrations used. ZnO–CTS–glycerol NPs and ZnO–CTS–CA NPs prepared by nano spray drying and precipitation method, respectively, showed maximum biofilm inhibition at concentration of 0.156 mg/ml. No significant differences (p > 0.05) with respect to M. luteus and S. aureus biofilms inhibition were observed with NPs prepared by two different methods.
ZnO–CTS NPs showed higher biofilm inhibition activity against both bacterial strains as compared to micro particles of ZnO and CTS. Higher biofilm inhibition activity was achieved with lower concentration of NPs (ranging from 0.625 to 0.156 mg/ml) as compared to micro particles (ZnO and CTS powder) which exerted higher biofilm inhibition activity at higher concentrations of 5 mg/ml.
Micrococcus luteus and Staphylococcus aureus represent two of the most common opportunistic pathogens, due to their frequent incidence in the etiology of the community-acquired and nosocomial infections and to their high rates of natural and acquired resistance to different antimicrobial agents including the widely used antibiotics. The clinical incidence of these bacterial strains is augmented due to their ability to colonize various industrial settings and the cellular as well as the inert substrates, such as medical devices. Both these strains are responsible for the formation of biofilms on medical devices and are associated with various chronic infections which are very hard to treat and often are responsible for severe outcome [41]. In the present study, the inhibitory effect on biofilm could be explained by the potential inhibitory effect of the ZnO NPs on the bacterial exopolysaccharides secretion. Moreover, both ZnO and CTS showed antimicrobial properties. These results are accounting for the potential use of ZnO–CTS NPs in the prevention of the bacterial biofilm development on prosthetic devices, as well as for the design of new antiseptics and disinfectants with efficient protective action against bacterial colonization of tissue and inert surfaces. Further, different types of formulations can be developed for these NPs through encapsulation, powder and hydrogels to suit different application needs.
Conclusions
The study demonstrated the facile synthesis of ZnO–CTS nanoparticles stabilized with different organic compounds. The synthesis of NPs was carried out by two different methods: (1) nano spray drying and; (2) precipitation method. The size distribution analysis, UV–Vis spectrum and SEM monographs of ZnO–CTS NPs indicated that NPs prepared in the presence of stabilizers agglomerated less and dispersed better in water as compared to ZnO and ZnO–CTS NPs without stabilizers. Both the synthesis methods were simple and were devoid of any chemical usage. However, the ratios of ZnO, CTS and stabilizer need to be further optimized for uniform size, shape and better dispersed NPs. The ZnO–CTS NPs showed significant antimicrobial activity and biofilm inhibition activity against M. luteus and S. aureus. The study indicated the promising potential of ZnO–CTS NPs as antimicrobial and biofilm inhibition agents.
Abbreviations
- CA:
-
Citric acid
- CTS:
-
Chitosan
- NPs:
-
Nanoparticles
- SEM:
-
Scanning electron microscope
- PDI:
-
Polydispersity index
References
Wei, A., Sun, X.W., Wang, J.X., Lei, Y., Cai, X.P., Li, C.M., Dong, Z.L., Huang, W.: Enzymatic glucose biosensor based on ZnO nano rods grown by hydrothermal decomposition. Appl. Phys. Lett. 89(12), 123902 (2006)
Wang, J.X., Sun, X.W., Wei, A., Lei, Y., Cai, X.P., Li, C.M., Dong, Z.L.: Zinc oxide nano comb biosensor for glucose detection. Appl. Phys. Lett. 88(23), 233106–233108 (2006)
Singh, S.P., Arya, S.K., Pandey, P., Malhotra, B.D., Saha, S., Sreenivas, K., Gupta, V.: Cholesterol biosensor based on sputtered zinc oxide nano porous thin film. Appl. Phys. Lett. 91(1–3), 063901 (2007)
Cheng, J.P., Zhang, X.B., Tao, X.Y., Lu, H.M., Luo, Z.Q., Liu, F.: Fine-tuning the synthesis of ZnO nanostructures by an alcohol thermal process. J. Phys. Chem. B 110(21), 10348–10353 (2006)
Wahab, R., Ansari, S.G., Kim, Y.S., Song, M., Shin, H.S.: The role of pH variation on the growth of zinc oxide nanostructures. Appl. Surf. Sci. 255(9), 4891–4896 (2009)
Zhang, J., Sun, L., Yin, J., Su, H., Liao, C., Yan, C.: Control of ZnO morphology via a simple solution route. Chem. Mater. 14(10), 4172–4177 (2002)
Ayudhya, S.K.N., Tonto, P., Mekasuwandumrong, O., Pavarajarn, V., Praserthdam, P.: Solvothermal synthesis of ZnO with various aspect ratios using organic solvents. Cryst. Growth Des. 6(11), 2446–2450 (2006)
Bitenc, M., Podbrscek, P., Orel, Z.C., Cleveland, M.A., Paramo, J.A., Peters, R.M., Strzhemechny, Y.M.: Correlation between morphology and defect luminescence in precipitated ZnO nanorod powders. Cryst. Growth Des. 9(2), 997–1001 (2009)
Ozgur, U., Alivov, Y.I., Liu, C., Teke, A., Reshchikov, M.A., Dogan, S., Avrutin, V., Cho, S.J., Morkoc, H.: A comprehensive review of ZnO materials and devices. J. Appl. Phys. 98(4), 041301–041303 (2005)
Kim, J.Y., Osterloh, F.E.: ZnO–CdSe nanoparticles clusters as directional photo emitters with tunable wavelength. J. Am. Chem. Soc. 127(29), 10152–10153 (2005)
Hubbard, N.B., Culpepper, M.L., Howell, L.L.: Actuators for micro positioners and nano positioners. Appl. Mech. Rev. 59(1–6), 324–334 (2006)
Lee, H.J., Yeo, S.Y., Jeong, S.H.: Antibacterial effect of nano sized silver colloidal solution on textile fabrics. J. Mater. Sci. 38(10), 2199–2204 (2003)
Wang, L., Muhammed, M.: Synthesis of zinc oxide nanoparticles with controlled morphology. J. Mater. Chem. 9(11), 2871–2878 (1999)
Xu, H.Y., Wang, H., Zhang, Y.C.: Hydrothermal synthesis of zinc oxide powders with controllable morphology. Ceramics Inter 30(1), 93–97 (2004)
Tani, T., Mdler, L., Pratsinis, S.E.: Homogeneous ZnO nanoparticles by flame spray pyrolysis. J Nanoparticle Res 4(4), 337–343 (2002)
Li, L.H., Deng, J.C., Deng, H.R., Liu, Z.L., Xin, L.: Synthesis and characterization of chitosan/ZnO nanoparticle composite membranes. Carbohyd Res 345(8), 994–998 (2010)
Agnihotri, S.A., Mallikarjuna, N.N., Aminabhavi, T.M.: Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Controlled Release 100(1), 5–28 (2004)
Kim, S.K., Rajapakse, N.: Enzymatic production and biological activities of chitosan oligosaccharides (COS): a review. Carbohyd Polym 62(4), 357–368 (2005)
Muzzarelli, R.A.A., Sipos, L.: Chitosan for the collection from seawater of naturally occurring zinc, cadmium, lead and copper. Talanta 18(9), 853–858 (1971)
Posthumus, W., Magusin, P.C.M., Zijp, J.C.M.B., Tinnemans, A.H.A., Linde, R.: Surface modification of oxidic nanoparticles using 3-methacryloxypropyl-trimethoxysilane. J. Coll. Interf. Sci. 269, 109–116 (2004)
Tang, E., Cheng, G., Ma, X., Pang, X., Zhao, Q.: Surface modification of ZnO nanoparticles by PMAA and its dispersion in aqueous system. Appl. Surf. Sci. 252(14), 5227–5232 (2006)
Ryan, K.J., Ray, C.G. (ed.): Sherris Medical Microbiology (4th ed). McGraw Hill, New York, ISBN 0-8385-8529-9 (2004)
Bowersox, J.: Experimental staph vaccine broadly protective in animal studies. NIH. Archived from the original on 5 May 2007(1999)
Limban, C., Marutescu, L., Chifiriuc, M.C.: Synthesis, spectroscopic properties and antipathogenic activity of new thiourea derivatives. Molecules 16(9), 7593–7607 (2011)
Olar, R., Badea, M., Marinescu, D., Chifiriuc, M.C., Bleotu, C., Grecu, M.N., Iorgulescu, E.M., Bucur, M., Lazar, A., Finaru, A.: Prospects for new antimicrobials based on N,N dimethyl biguanide complexes as effective agents on both planktonic and adhered microbial strains. Eur. J. Med. Chem. 45, 2868–2875 (2010)
Pesika, N.S., Stebe, K.J., Searson, P.C.: Determination of the particle size distribution of quantum nano crystals from absorbance spectra. Adv. Matter. 15(15), 1289–1296 (2003)
Ji, X., Song, X., Li, J., Bai, Y., Yang, W., Peng, X.: Size control of gold nano crystals in citrate reduction: the third role of citrate. J. Am. Chem. Soc. 129(45), 13939–13948 (2007)
Jiang, X.C., Chen, C.Y., Chen, W.M., Yu, A.B.: Role of citric acid in the formation of silver nano plates through a synergistic reduction approach. Langmuir 26(6), 4400–4408 (2010)
Jin, R., Cao, Y., Metraux, G.S., Schatz, G.C., Mirkin, C.A.: Controlling anistropic nanoparticle growth through plasmon excitation. Nature 425, 487–490 (2003)
Jin, R., Cao, Y., Mirkin, C.A., Kelly, K.L., Schatz, G.C., Zheng, J.G.: Photo induced conversion of silver nanospheres to nanoprisms. Sci 294(5548), 1901–1903 (2001)
Nair, S., Sasidharan, A., Divya Rani, V.V., Menon, D., Nair, S., Manzoor, K., Raina, S.: Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Med. 20(S1), 235–241 (2009)
Adams, L.K., Lyon, D.Y., Alvarez, P.J.J.: Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 40(19), 3527–3532 (2006)
Brayner, R., Ferrari-Iliou, R., Brivois, N., Djediat, S., Benedetti, M.F., Fiévet, F.: Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 6(4), 866–870 (2006)
Colon, G., Ward, B.C., Webster, T.J.: Increased osteoblast and decreased staphylococcus epidermidis functions on nanophase ZnO and TiO2. J. Biomed. Mater. Res. A78, 595–604 (2006)
Jeng, H.A., Swanson, J.: Toxicity of metal oxide nanoparticles in mammalian cells. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 41(12), 2699–2711 (2006)
Yang, Z., Xie, C.: Zn2+ release from zinc and zinc oxide particles in simulated uterine solution. Coll. Surf. B Biointerf. 47(2), 140–145 (2006)
Zhang, L., Jiang, Y., Ding, Y., Povey, M., York, D.: Investigation into the antibacterial behavior of suspensions of ZnO nanoparticles (ZnO nano fluids). J. Nano. Part. Res. 9(3), 479–489 (2007)
Reddy, K.M., Feris, K., Bell, J., Wingett, D.G., Hanley, C., Punnoose, A.: Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 90(213902), 2139021–2139023 (2007)
Yamamoto, O.: Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 3(7), 643–646 (2001)
Sawai, J., Shouji, S., Igarashi, H., Hashimoto, A., Kokugan, T., Shimizu, M., Kojima, H.: Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J. Ferment. Bioeng. 86(5), 521–522 (1998)
Donlan, R.M., Costerton, J.W.: Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15, 167–193 (2002)
Acknowledgments
The authors are sincerely thankful to the Natural Sciences and Engineering Research Council of Canada (Discovery Grant 355254) and Initiative Inde 2010 (MELS) for financial support. The views or opinions expressed in this article are those of the authors.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd.Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Dhillon, G.S., Kaur, S. & Brar, S.K. Facile fabrication and characterization of chitosan-based zinc oxide nanoparticles and evaluation of their antimicrobial and antibiofilm activity. Int Nano Lett 4, 107 (2014). https://doi.org/10.1007/s40089-014-0107-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40089-014-0107-6