Abstract
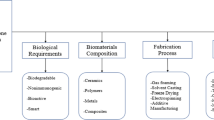
The systematic review refers to the advancement of Rapid Prototyped facilitated scaffolding for regeneration of tissue from the bone. Bone tissue is a mineralized tissue consisting of cortical bones and cancellous bones with two types of hard tissue structure. The bone's dense outer shell, often referred to as the compact bone, it makes up nearly 80% of the cortical bone's skeletal mass, While the cancellous part shares the remaining 20%. Many different forms of cells & substances are participating in the bone remodeling mechanism. Bone remodeling is a highly intricate mechanism in which osteocytes, osteoblasts, and osteoclasts work together to restore old bone with new bone. Osteoclasts are in charge of ageing bone resorption, while osteoblasts are in charge of new bone production. Osteocytes serve as mechanosensors and coordinators during bone remodeling. When used in combination with traditional methods, gas foaming, particulate leaching, fiber bonding, phase separation, lamination of membranes, melting, solvent casting, and freeze drying have been used to manufacture tissue-engineered structures. Although these scaffold manufacturing techniques do not help designers optimize internal architecture that could lead to the development of biological processes and tissues, they do have other benefits. In Rapid Prototyping (RP), complicated and convoluted structures can be successfully produced. Using “RP” in bone scaffold generation results in enhanced strength, in-vitro, and in-vivo cell culture, helps in increasing the capacity for patient-specific bone scaffolds design. This study evaluated the advantages of RP/imaging/CAD/CAM in making scaffolds for bone tissue reconstruction, and it is beneficial to those patients who were otherwise unable to be treated because of the conventional methods. The study explores the possible effects of RP on organ transplantation.









Similar content being viewed by others
Abbreviations
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
- ALP:
-
Alkaline phosphatase
- ADMSCs:
-
Adipose tissue-derived mesenchymal stem cells
- BG:
-
Bioactive glass
- bFGF:
-
Basic fibroblast growth factor
- 3T3:
-
Fibroblasts cells
- BMP-2:
-
Bone morphogenic protein-2
- BMSCs:
-
Bone marrow stromal cells
- BSA:
-
Bovine serum albumin
- CPC:
-
Calcium phosphate cement
- CSH:
-
Calcium phosphate cement
- CT:
-
Computed tomography
- dECM:
-
Decellularized extracellular matrix
- DMSO:
-
Dimethyl sulfoxide
- DNA:
-
Deoxyribonucleic acid
- EBM:
-
Electron beam melting
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- EPCs:
-
Endothelial progenitor cells
- FHAp:
-
Fluorhydroxyaptite
- FDM:
-
Fused deposition modelling
- GF:
-
Growth factor
- GO:
-
Graphene oxide
- HA:
-
Hyaluronic acid
- HAp:
-
Hydroxyapatite
- hADSCs:
-
Human adipose drived stem cells
- hAVICs:
-
Human aortic valvular interstitial cells
- hBMSCs:
-
Human bone marrow derived stem cells
- hDFCs:
-
Human dental follicle cells
- hEKCs:
-
Human embryonic kidney cells
- hFOBCs:
-
Human fetal osteoblasts cells
- hNDFs:
-
Human neonatal dermal fibroblasts
- hTMSCs:
-
Human inferior turbinate tissue derived mesenchymal stromal cells
- LBM:
-
Laser beam melting
- MBG:
-
Mesoporous bioactive glass
- MC3T3-E1:
-
Osteoblast precursor cell line
- MG-63:
-
Human osteosarcoma cell line
- MRI:
-
Magnetic resonance imagining
- MSC:
-
Mesenchymal stem cells
- MWCNTs:
-
Multi-wall carbon nanotubes
- NHEKs:
-
Normal human epidermal keratinocytes
- oMSCs:
-
Ovine mesenchymal stem cells
- PAA:
-
Poly(acrylic acid)
- PCL:
-
Poly(caprolactone)
- PEG:
-
Poly(ethylene oxide)
- PEI:
-
Polyethylenelmine
- PEO:
-
Poly(ethylene oxide)
- PES:
-
Polyethersulphone
- PET:
-
Polyethylene terephthalate
- PGA:
-
Poly(glycolic acid)
- PGF:
-
Platelet derived growth factor
- PHB:
-
Polyhydroxybutyrate
- PLC:
-
Poly(L-lactide caprolactone)
- PLGA:
-
Poly-lactic-glycolic acid
- PLA:
-
Poly lactic acid
- PMMA:
-
Poly(methyl methacrylate)
- PP:
-
Polypropylene
- PU:
-
Polyurethane
- PVA:
-
Polyvinyl alcohol
- PVAc:
-
Polyvinyl acetate
- rBMSC:
-
Rat bone mesenchymal stem cells
- rCCs:
-
Rabbit corneal cells
- rGO:
-
Reduced graphene oxide
- RNA:
-
Ribonucleic acid
- SDSCs:
-
Synovium derived stem cells
- SiHAp:
-
Silicate containing hydroxyapatite
- SLA:
-
Stereolithography
- SLS:
-
Selective laser sintering
- TPU:
-
Thermoplastic polyurethane
- VEGF:
-
Vascular endothelial growth factor
References
S. Bose, S. Vahabzadeh, A. Bandyopadhyay, Bone tissue engineering using 3D printing. Mater. Today 16(12), 496–504 (2013). https://doi.org/10.1016/j.mattod.2013.11.017
J. Wolff et al., Biology of bone tissue: structure, function, and factors that influence bone cells. Tissue Eng. 7(6), 679–689 (2015). https://doi.org/10.1302/2046-3758.73.BJR-2017-0270.R1
M. Ansari, Bone tissue regeneration: biology, strategies and interface studies. Prog. Biomater. 8(4), 223–237 (2019). https://doi.org/10.1007/s40204-019-00125-z
J. Wolff et al., Design and 3D printing of scaffolds and tissues. J. R. Soc. Interface 7(1), 1832–1839 (2003). https://doi.org/10.1016/j.tibtech.2004.05.005
S. Yang, K.F. Leong, Z. Du, C.K. Chua, The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 7(6), 679–689 (2001). https://doi.org/10.1089/107632701753337645
J. Wolff et al., The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 7(6), 679–689 (2015). https://doi.org/10.1302/2046-3758.73.BJR-2017-0270.R1
J. Wolff et al., Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Tissue Eng. 7(6), 679–689 (2018). https://doi.org/10.1302/2046-3758.73.BJR-2017-0270.R1
H.T. Halonen, T.O. Ihalainen, L. Hyväri, S. Miettinen, J.A.K. Hyttinen, Cell adhesion and culture medium dependent changes in the high frequency mechanical vibration induced proliferation, osteogenesis, and intracellular organization of human adipose stem cells. J Mech Behav Biomed Mater (2020). https://doi.org/10.1016/j.jmbbm.2019.103419
R. Landers, A. Pfister, U. Hübner, H. John, R. Schmelzeisen, R. Mülhaupt, Fabrication of soft tissue engineering scaffolds by means of rapid prototyping techniques. J. Mater. Sci. 37(15), 3107–3116 (2002). https://doi.org/10.1023/A:1016189724389
P.S. Sapkal, S. Jaiswal, A.M. Kuthe, Rapid prototyping assisted scaffold fabrication for bone tissue regeneration. J. Mater. Sci. Res. 5(4), 79 (2016). https://doi.org/10.5539/jmsr.v5n4p79
T.D. Ngo, A. Kashani, G. Imbalzano, K.T.Q. Nguyen, D. Hui, Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Compos Part B Eng (2018). https://doi.org/10.1016/j.compositesb.2018.02.012
B.K. Gu, D.J. Choi, S.J. Park, M.S. Kim, C.M. Kang, C.H. Kim, 3-Dimensional bioprinting for tissue engineering applications. Biomater. Res. 20(1), 1–8 (2016). https://doi.org/10.1186/s40824-016-0058-2
R. Landers et al., Bio-rapid-prototyping of tissue engineering scaffolds and the process-induced cell damage. Engineering 59(1), 3635–3640 (2018). https://doi.org/10.2174/1874070701812010241
N. Sultana, Mechanical and biological properties of scaffold materials (Elsevier Ltd, Armsterdam, 2018)
T.T. Tran, Z.A. Hamid, K.Y. Cheong, A review of mechanical properties of scaffold in tissue engineering: aloe vera composites. J Phys Conf Ser (2018). https://doi.org/10.1088/1742-6596/1082/1/012080
G. Turnbull et al., 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 3(3), 278–314 (2018). https://doi.org/10.1016/j.bioactmat.2017.10.001
K.F. Leong, C.M. Cheah, C.K. Chua, Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 24(13), 2363–2378 (2003). https://doi.org/10.1016/S0142-9612(03)00030-9
D.W. Hutmacher, Scaffolds in tissue engineering bone and cartilage. Biomater. Silver Jubil. Compend. 21, 175–189 (2000). https://doi.org/10.1016/B978-008045154-1.50021-6
B. Thavornyutikarn, N. Chantarapanich, K. Sitthiseripratip, G.A. Thouas, Q. Chen, Bone tissue engineering scaffolding: computer-aided scaffolding techniques. Prog Biomater 3(2–4), 61 (2014)
S.W. Suh et al., Effect of different particles on cell proliferation in polymer scaffolds using a solvent-casting and particulate leaching technique. ASAIO J. 48(5), 460–464 (2002). https://doi.org/10.1097/00002480-200209000-00003
Q. Lv, Q.L. Feng, Preparation of 3-D regenerated fibroin scaffolds with freeze drying method and freeze drying/foaming technique. J. Mater. Sci. Mater. Med. 17(12), 1349–1356 (2006). https://doi.org/10.1007/s10856-006-0610-z
K.W. Lee, S. Wang, B.C. Fox, E.L. Ritman, M.J. Yaszemski, L. Lu, Poly(propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: effects of resin formulations and laser parameters. Biomacromol 8(4), 1077–1084 (2007). https://doi.org/10.1021/bm060834v
S.A. Poursamar, J. Hatami, A.N. Lehner, C.L. Da Silva, F.C. Ferreira, A.P.M. Antunes, Gelatin porous scaffolds fabricated using a modified gas foaming technique: characterisation and cytotoxicity assessment. Mater. Sci. Eng. C 48, 63–70 (2015). https://doi.org/10.1016/j.msec.2014.10.074
S. Lohfeld, M.A. Tyndyk, S. Cahill, N. Flaherty, V. Barron, P.E. McHugh, A method to fabricate small features on scaffolds for tissue engineering via selective laser sintering. J. Biomed. Sci. Eng. 3(02), 138–147 (2010). https://doi.org/10.4236/jbise.2010.32019
A. Chládová, J. Wiener, J.M. Luthuli, V. Zajícová, Dyeing of glass fibres by the sol gel method. Autex Res. J. 11(1), 18–23 (2011)
M. Javaid, A. Haleem, Additive manufacturing applications in medical cases: a literature based review. Alexandria J. Med. 54(4), 411–422 (2018). https://doi.org/10.1016/j.ajme.2017.09.003
Ó. Libardo et al., An algorithm to optimize the micro-geometrical dimensions of scaffolds with spherical pores. Materials. 13(18), 4062 (2020)
M. Bahraminasab, Challenges on optimization of 3D - printed bone scaffolds. Biomed. Eng. Online (2020). https://doi.org/10.1186/s12938-020-00810-2
S. Karuppudaiyan, D.K.J. Singh, Design of scaffold with controlled internal architecture using Fused Deposition Modeling (FDM). IJEAT 9(1), 2764–2768 (2016)
A. Baliga and S. Borkar, “A Review of the 3D Designing of Scaffolds for Tissue Engineering with a Focus on Keratin Protein,” no. September, 2016, doi: https://doi.org/10.20944/preprints201609.0091.v1.
M.H. Jalil, M. Todo, Development and characterization of gear shape porous scaffolds using 3D printing technology. IJBBB 7(2), 74–83 (2017)
J. An, J.E.M. Teoh, R. Suntornnond, C.K. Chua, Design and 3D printing of scaffolds and tissues. Engineering 1(2), 261–268 (2015). https://doi.org/10.15302/J-ENG-2015061
S. Mohanty et al., Fabrication of scalable tissue engineering scaffolds with dual-pore microarchitecture by combining 3D printing and particle leaching. Mater. Sci. Eng. C 61, 180–189 (2016). https://doi.org/10.1016/j.msec.2015.12.032
X. Du, Y. Zhu, 3D printing of ceramic-based scaffolds for bone tissue engineering: an overview. J Mater Chem B (2018). https://doi.org/10.1039/c8tb00677f
H. Seitz, W. Rieder, S. Irsen, B. Leukers, C. Tille, Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res (2005). https://doi.org/10.1002/jbm.b.30291
F. Curti et al., “Printing Using Wollastonite – Gelatin Inks,” pp. 8–11
W.Y. Yeong, C.K. Chua, K.F. Leong, M. Chandrasekaran, Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol. 22(12), 643–652 (2004). https://doi.org/10.1016/j.tibtech.2004.10.004
S. Yang, K.F. Leong, Z. Du, C.K. Chua, The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Eng. 8(1), 1–11 (2002). https://doi.org/10.1089/107632702753503009
L. Moroni, J.R. De Wijn, C.A. Van Blitterswijk, 3D fiber-deposited scaffolds for tissue engineering: influence of pores geometry and architecture on dynamic mechanical properties. Biomaterials (2006). https://doi.org/10.1016/j.biomaterials.2005.07.023
M. T. Arafat, M. M. Savalani, and I. Gibson, “Improving the mechanical properties in tissue engineered scaffolds,” pp. 6–9, 2016
Z. Fang, B. Starly, W. Sun, Computer-aided characterization for effective mechanical properties of porous tissue scaffolds. Comput-Aid Design (2005). https://doi.org/10.1016/j.cad.2004.04.002
M. Schumacher, U. Deisinger, R. Detsch, G. Ziegler, Indirect rapid prototyping of biphasic calcium phosphate scaffolds as bone substitutes : influence of phase composition, macroporosity and pore geometry on mechanical properties. J Mater Sci Mater Med (2010). https://doi.org/10.1007/s10856-010-4166-6
P.H. Warnke et al., Rapid prototyping : porous titanium alloy scaffolds roduced by selective laser melting for bone tissue engineering. Tissue Eng Part C: Meth 15(2), 115–124 (2009)
P. Taylor, M. E. Hoque, D. W. Hutmacher, W. Feng, S. Li, and M. Huang, “Journal of Biomaterials Science , Fabrication using a rapid prototyping system and in vitro characterization of PEG-PCL-PLA scaffolds for tissue engineering,” no. November 2014, pp. 37–41, 2012, doi: https://doi.org/10.1163/156856205774576709.
Z. Duan et al., A three-dimensional model of the yeast genome. Nature 465(7296), 363–367 (2010). https://doi.org/10.1038/nature08973
S. Naghieh, M.R. KaramoozRavari, M. Badrossamay, E. Foroozmehr, M. Kadkhodaei, Numerical investigation of the mechanical properties of the additive manufactured bone scaffolds fabricated by FDM: the effect of layer penetration and post-heating. J Mech Behav Biomed Mater (2016). https://doi.org/10.1016/j.jmbbm.2016.01.031
X. Chen, Z. Wang, N. Duan, G. Zhu, E.M. Schwarz, C. Xie, Osteoblast–osteoclast interactions. Connect. Tissue Res. 59(2), 99–107 (2018). https://doi.org/10.1080/03008207.2017.1290085
S. Park, G. Kim, Y. Chul, 3D polycaprolactone scaffolds with controlled pore structure using a rapid prototyping system. J Mater Sci: Mater Med (2009). https://doi.org/10.1007/s10856-008-3573-4
L. Liu, Z. Xiong, Y. Yan, Y. Hu, R. Zhang, and S. Wang, “Porous morphology , porosity , mechanical properties of poly (a -hydroxy acid)– tricalcium phosphate composite scaffolds fabricated by low-temperature deposition,” 2007, doi: https://doi.org/10.1002/jbm.a.
J.M. Williams et al., Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials (2005). https://doi.org/10.1016/j.biomaterials.2004.11.057
M. Domingos, A. Gloria, L. Ambrosio, Effect of process parameters on the morphological and mechanical properties of 3D Bioextruded poly (1 -caprolactone) scaffolds. Rapid Prototyp J (2012). https://doi.org/10.1108/13552541211193502
S. Heo et al., Fabrication and characterization of novel nano- and micro-HA / PCL composite scaffolds using a modified rapid prototyping process. J Biomed Mater Res (2008). https://doi.org/10.1002/jbm.a.31726
M.V. Cabañas, J.L. Paris, D. Lozano, Fabrication of novel Si-doped Hydroxyapatite / Gelatine scaffolds by rapid prototyping for drug delivery and bone regeneration. ACTA Biomater (2014). https://doi.org/10.1016/j.actbio.2014.12.021
H. Yun, S. Kim, E. Kyun, Bioactive glass – poly (ε -caprolactone) composite scaffolds with 3 dimensionally hierarchical pore networks. Mater. Sci. Eng. C 31(2), 198–205 (2011). https://doi.org/10.1016/j.msec.2010.08.020
N. Sudarmadji, J.Y. Tan, K.F. Leong, C.K. Chua, Y.T. Loh, Acta Biomaterialia Investigation of the mechanical properties and porosity relationships in selective laser-sintered polyhedral for functionally graded scaffolds. Acta Biomater. 7(2), 530–537 (2011). https://doi.org/10.1016/j.actbio.2010.09.024
N. W. Pensa et al., “3D printed mesh reinforcements enhance the mechanical properties of electrospun scaffolds,” pp. 1–7, 2019
G. Kim, J. Son, S. Park, and W. Kim, “Hybrid Process for Fabricating 3D Hierarchical Scaffolds Combining Rapid Prototyping and Electrospinning,” pp. 1577–1581, 2008, doi: https://doi.org/10.1002/marc.200800277.
G.V. Salmoria, P. Klauss, R.A. Paggi, L.A. Kanis, A. Lago, Structure and mechanical properties of cellulose based scaffolds fabricated by selective laser sintering. Polym. Test. 28(6), 648–652 (2009). https://doi.org/10.1016/j.polymertesting.2009.05.008
N. Sahai and R. P. Tewari, “Characterization o f e ffective m echanical s trength o f c hitosan p orous t issue s caffolds using,” vol. 2, no. 1, pp. 21–28, 2015.
L. Lin, A. Tong, H. Zhang, Q. Hu, and M. Fang, “The Mechanical Properties of Bone Tissue Engineering Scaffold Fabricating Via Selective Laser Sintering,” pp. 146–152, 2007.
K.C. Ang, K.F. Leong, C.K. Chua, fabricated porous structures investigation of the mechanical properties and porosity relationships in fused deposition modelling-fabricated porous structures. Rapid Prototyp J (2006). https://doi.org/10.1108/13552540610652447
P. Taylor et al., “Virtual and Physical Prototyping Mechanical and in vitro evaluations of composite PLDLLA / TCP scaffolds for bone engineering Mechanical and in vitro evaluations of composite PLDLLA/TCP scaffolds for bone engineering,” no. November 2014, pp. 37–41, 2008, doi: https://doi.org/10.1080/17452750802551298.
Q. Liu, M.C. Leu, S.M. Schmitt, Rapid prototyping in dentistry: technology and application. Int. J. Adv. Manuf. Technol. 29(3–4), 317–335 (2006). https://doi.org/10.1007/s00170-005-2523-2
M. Askari, M. AfzaliNaniz, M. Kouhi, A. Saberi, A. Zolfagharian, M. Bodaghi, Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: a comprehensive review with focus on advanced fabrication techniques. Biomater Sci. (2021). https://doi.org/10.1039/d0bm00973c
M.A. Skylar-Scott et al., Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. (2019). https://doi.org/10.1126/sciadv.aaw2459
M.B. Mawale, A.M. Kuthe, S.W. Dahake, Additive layered manufacturing: State-of-the-art applications in product innovation. Concurr. Eng. Res. Appl. 24(1), 94–102 (2016). https://doi.org/10.1177/1063293X15613111
S. Ji, M. Guvendiren, 3D printedwavy scaffolds enhance mesenchymal stem cell osteogenesis. Micromachines (2020). https://doi.org/10.3390/mi11010031
M. Rezai, F. Fahimipour, E. Dashtimoghadam, H. Nokhbatolfoghahaei, L. Tayebi, A. Khojasteh, Bioprinting Osteogenic differentiation of adipose-derived mesenchymal stem cells using 3D-Printed PDLLA / β -TCP nanocomposite scaffolds. Bioprinting (2021). https://doi.org/10.1016/j.bprint.2020.e00117
E. De Giglio et al., Multi-compartment scaffold fabricated via 3D-printing as in vitro co-culture osteogenic model. Sci Rep (2018). https://doi.org/10.1038/s41598-018-33472-1
H.A.M. Abu et al., strong 3D bioprinted scaffolds for bone repair. J Controll Release (2020). https://doi.org/10.1016/j.jconrel.2020.06.035
H. Kim, C. Bae, Y. Kook, W. Koh, K. Lee, M.H. Park, Mesenchymal stem cell 3D encapsulation technologies for biomimetic microenvironment in tissue regeneration. Stem Cell Res Ther (2019). https://doi.org/10.1186/s13287-018-1130-8
S. Cells et al., The stiffness and structure of three-dimensional printed hydrogels direct the differentiation of mesenchymal. Tissue Eng Part A (2015). https://doi.org/10.1089/ten.tea.2014.0231
N. Carolina, “F et al. and N EONATAL S TEM C ELLS Bioprinted Amniotic Fluid-Derived Stem Cells Accelerate Healing of Large Skin Wounds,” pp. 792–802, 2012.
D. Filipa, D. Campos, A. Blaeser, K. Buellesbach, K.S. Sen, Bioprinting organotypic hydrogels with improved mesenchymal stem cell remodeling and mineralization properties for bone tissue engineering. Adv Healthc Mater (2016). https://doi.org/10.1002/adhm.201501033
M. Du, Q. Meng, C. Zhang, and W. Heran, “3D bioprinting of BMSC-laden methacrylamide gelatin scaffolds with CBD- BMP2-collagen microfibers My IOPscience 3D bioprinting of BMSC-laden methacrylamide gelatin scaffolds with CBD-BMP2-collagen microfibers This content has been downloaded from IOPscien,” no. December, 2015, doi: https://doi.org/10.1088/1758-5090/7/4/044104
R. Levato, J. Visser, J.A. Planell, E. Engel, Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication (2014). https://doi.org/10.1088/1758-5082/6/3/035020
P. Apelgren et al., Chondrocytes and stem cells in 3D-bioprinted structures create human cartilage in vivo. PLoS ONE (2017). https://doi.org/10.1371/journal.pone.0189428
B.S. Kim, Y.W. Kwon, J. Kong, G.T. Park, G. Gao, B.S. Kim, 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials (2018). https://doi.org/10.1016/j.biomaterials.2018.03.040
R. Gaebel et al., Biomaterials patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials 32(35), 9218–9230 (2011). https://doi.org/10.1016/j.biomaterials.2011.08.071
S.A. Grafts et al., Laser printing of stem cells for biofabrication. Tissue Eng Part C Meth (2011). https://doi.org/10.1089/ten.tec.2010.0359
M. Sladkova, G.M. De Peppo, Bioreactor systems for human bone tissue engineering. Processes (2014). https://doi.org/10.3390/pr2020494
J. Rauh, F. Milan, K.P. Günther, M. Stiehler, Bioreactor systems for bone tissue engineering. Tissue Eng. - Part B Rev. 17(4), 263–280 (2011). https://doi.org/10.1089/ten.teb.2010.0612
C.C. Liao, H.Y. Wang, S.H. Chuang, M.L. Shih, C.C. Liu, Enhancing knowledge management for R&D innovation and firm performance: an integrative view. Afr J. Bus. Manag. 4(14), 3026–3038 (2010)
A.J. El Haj, S.H. Cartmell, Bioreactors for bone tissue engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. (2010). https://doi.org/10.1243/09544119JEIM802
M. Nishi, R. Matsumoto, J. Dong, T. Uemura, Engineered bone tissue associated with vascularization utilizing a rotating wall vessel bioreactor. J Biomed Mater Res Part A (2013). https://doi.org/10.1002/jbm.a.34340
A. KoçDemir, A.E. Elçin, Y.M. Elçin, Osteogenic differentiation of encapsulated rat mesenchymal stem cells inside a rotating microgravity bioreactor: in vitro and in vivo evaluation. Cytotechnology (2018). https://doi.org/10.1007/s10616-018-0230-8
B. Carpentier, P. Layrolle, C. Legallais, Bioreactors for bone tissue engineering. Int. J. Artif. Organs 34(3), 259–270 (2011). https://doi.org/10.5301/IJAO.2011.6333
F. Yasmin, X. Chen, Role of bioreactors in regeneration of articular cartilage. Sens Mater. 28(10), 1129–1140 (2016)
P. Godara, C.D. Mcfarland, R.E. Nordon, Design of bioreactors for mesenchymal stem cell tissue engineering. J Chem Tech Biotech: Int Res Process Environ, Clean Tech (2008). https://doi.org/10.1002/jctb
A. Nazempour, B.J. Van Wie, A flow perfusion bioreactor with controlled mechanical stimulation: application in cartilage tissue engineering and beyond. J Stem Cell Ther Transplant 2, 15–34 (2018)
D. Gaspar, D.A. Gaspar, V. Gomide, F.J. Monteiro, The role of perfusion bioreactors in bone tissue engineering. Biomatter (2014). https://doi.org/10.4161/biom.22170
M. Pleasant, I. Bone, L. Nail, Review mechanistic role of perfusion culture on bone regeneration. J Biosci (2019). https://doi.org/10.1007/s12038-018-9827-5
J. Salgado, O.P. Coutinho, R.L. Reis, Bone tissue engineering: state of the art and future trends. Macromol Biosci (2004). https://doi.org/10.1002/mabi.200400026
A. Eltom, G. Zhong, A. Muhammad, Scaffold techniques and designs in tissue engineering functions and purposes: a review. Adv Mater Sci Eng (2019). https://doi.org/10.1155/2019/3429527
J.A. Mcgovern, M. Griffin, D.W. Hutmacher, Animal models for bone tissue engineering and modelling disease. Diseas Mod Mechan (2018). https://doi.org/10.1242/dmm.033084
K.C.R. Kolan, Y. Huang, J.A. Semon, M.C. Leu, “3D-printed biomimetic bioactive glass scaffolds for bone regeneration in rat calvarial defects. Int J Bioprint (2020). https://doi.org/10.18063/ijb.v6i2.274
P. Fomby et al., Stem cells and cell therapies in lung biology and diseases: conference report. Ann. Am. Thorac. Soc. 12(3), 181–204 (2010). https://doi.org/10.1002/term
P. Korn et al., 3D printing of bone grafts for cleft alveolar osteoplasty–In vivo Evaluation in a Preclinical Model. Front Bioeng Biotechnol (2020). https://doi.org/10.3389/fbioe.2020.00217
S. Hee, P. Dae, S. Park, J. Won, Scaffolds for bone tissue engineering fabricated from two different materials by the rapid prototyping technique PCL versus PLGA. J Mater Sci: Mater Med (2012). https://doi.org/10.1007/s10856-012-4738-8
F.E. Freeman, D.C. Browe, D. Pj, J. Nulty, S. Von Euw, W.L. Grayson, Biofabrication of multiscale bone extracellular matrix scaffolds for bone tissue engineering. Eur Cell Mater 38, 168–187 (2019)
T.B.F. Woodfield, J. Riesle, C.A. Van Blitterswijk, Rapid prototyping of anatomically shaped, tissue-engineered implants for restoring congruent articulating surfaces in small joints. Cell Proliferat (2009). https://doi.org/10.1111/j.1365-2184.2009.00608.x
Y. Guo et al., In vitro and in vivo study of 3D-printed porous tantalum scaffolds for repairing bone defects. ACS Biomater Sci Eng (2019). https://doi.org/10.1021/acsbiomaterials.8b01094
D.G. Tamay, T.D. Usal, A.S. Alagoz, D. Yucel, 3D and 4D printing of polymers for tissue engineering applications. Front Bioeng Biotechnol (2019). https://doi.org/10.3389/fbioe.2019.00164
M. Salah, L. Tayebi, K. Moharamzadeh, F.B. Naini, Three-dimensional bio-printing and bone tissue engineering: technical innovations and potential applications in maxillofacial reconstructive surgery. Maxillofac Plast Reconstr Surg (2020). https://doi.org/10.1186/s40902-020-00263-6
A. Manuscript, “Acced Muspt,” 2020
B.E. Grottkau, Z. Hui, Y. Yao, Y. Pang, Rapid fabrication of anatomically-shaped bone Sca ff olds using indirect 3D printing and perfusion techniques. IJMS (2020). https://doi.org/10.3390/ijms21010315
B.I. Oladapo, S.A. Zahedi, A.O.M. Adeoye, 3D printing of bone scaffolds with hybrid biomaterials. Compos Part B (2019). https://doi.org/10.1016/j.compositesb.2018.09.065
S.H. Irsen, S. Milz, C. Tille, M. Schieker, H. Seitz, Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J Mater Sci: Mater Med. 6, 1121–1124 (2005)
A.I. Pearce, R.G. Richards, S. Milz, E. Schneider, S.G. Pearce, Animal models for implant biomaterial research in bone: a review. Eur. Cells Mater. 13, 1–10 (2007). https://doi.org/10.22203/eCM.v013a01
A. Haleem, M. Javaid, R.H. Khan, R. Suman, 3D printing applications in bone tissue engineering. J Clin Orthop Trauma (2020). https://doi.org/10.1016/j.jcot.2019.12.002
P. Rider, ŽP. Kačarević, S. Alkildani, S. Retnasingh, R. Schnettler, M. Barbeck, Additive manufacturing for guided bone regeneration: a perspective for alveolar ridge augmentation. IJMS (2018). https://doi.org/10.3390/ijms19113308
S. Sharma, S.A. Goel, Three-dimensional printing and its future in medical world. J. Med. Res. Innov. 3(1), 1–8 (2018). https://doi.org/10.15419/jmri.141.Publication
Acknowledgements
This research received no financial support by any funding organization
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest, between the authors or between any organisation of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansari, A.I., Sheikh, N.A. Bone Tissue Regeneration: Rapid Prototyping Technology in Scaffold Design. J. Inst. Eng. India Ser. C 103, 1303–1324 (2022). https://doi.org/10.1007/s40032-022-00872-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40032-022-00872-2