Abstract
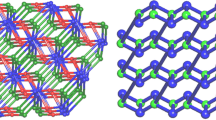
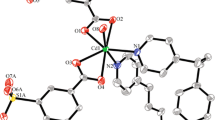
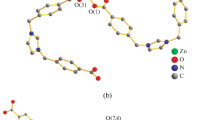
The bifurcated ligand 5-di(1H-benzo[d]imidazol-1-yl) benzonitrile (DBIBN) under solvothermal condition, undergoes basic as well as acid hydrolysis to the corresponding carboxylic acid (DBIBA). Four new coordination polymers; {[Cd(DBIBA)(CH3COO)]} n (1), {[Zn(DBIBA)(CH3COO)]⋅H2O} n (2), {[Zn(DBIBA)(OC2H5)]⋅H2O} n (3) and {[Co(DBIBA)(CH3COO)]⋅H2O} n (4) have been synthesized under solvothermal conditions. Compound 1 has a 3D structure with uninodal 4-connected crb/BCT topology with point symbol of {4.65}. In contrast, compounds 2–4 are 2D layered structures having uninodal 3-connected hcb net with a vertex symbol of {63}. The 2D layers are further assembled to an overall 3D supramolecular architecture with strong π–π interactions between layers. Further reinforcement of the 3D assembly occurs due to an array of H-bonding interactions with lattice water molecules present within the layers. All the compounds have been characterized by single X-ray crystallography, IR spectroscopy, TGA, PXRD measurements and elemental analysis. Although the metal-free DBIBN ligand exhibits fluorescence, it is quenched in the coordination polymers.
Graphical Abstract












Similar content being viewed by others
References
Ye BH, Tong ML, Chen XM (2005) Metal-organic molecular architectures with 2,2′-bipyridyl-like and carboxylate ligands. Coord Chem Rev 249:545
Biradha K, Sarkar M, Rajput L (2006) Crystal engineering of coordination polymers using 4,4′-bipyridine as a bond between transition metal atoms. Chem Commun 40:4169–4179
Hesham AH, Joaquin S, Christoph J (2008) Mixed-ligand coordination polymers from 1,2-bis(1,2,4-triazol-4-yl)ethane and benzene-1,3,5-tricarboxylate: trinuclear nickel or zinc secondary building units for three-dimensional networks with crystal-to-crystal transformation upon dehydration. J Dalton Trans 13:1734–1744
Hesham AH, Anke H, Henning AH, Christoph J (2009) Crystal structures and solid-state CPMAS 13C NMR correlations in luminescent zinc(II) and cadmium(II) mixed-ligand coordination polymers constructed from 1,2-bis(1,2,4-triazol-4-yl)ethane and benzenedicarboxylate. Dalton Trans 10:1742–1751
Li H, Eddaoudi M, O’Keeffe M, Yaghi OM (1999) Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 402:276
Kim J, Chen B, Reineke TM, Li H, Eddaoudi M, Moler DB, O’Keeffe M, Yaghi OM (2001) Assembly of metal−organic frameworks from large organic and inorganic secondary building units: new examples and simplifying principles for complex structures. J Am Chem Soc 123:8239
Barthelet K, Marrot J, Riou D, Férey G (2002) A breathing hybrid organic–inorganic solid with very large pores and high magnetic characteristics. Angew Chem Int Ed 41:281
Singh R, Ahmad M, Bharadwaj PK (2012) Coordination polymers of copper and zinc ions with a linear linker having imidazole at each end and an azo moiety in the middle: pedal motion, gas adsorption, and emission studies. Cryst Growth Des 12:5025
Rosi NL, Eckert J, Eddaoudi M, Vodak DT, Kim J, O’Keeffe M, Yaghi OM (2003) Hydrogen storage in microporous metal-organic frameworks. Science 300:1127
Dybtsev DN, Chun H, Kim K (2004) Rigid and flexible: a highly porous metal–organic framework with unusual guest-dependent dynamic behavior. Angew Chem Int Ed 43:5033
Wang ZQ, Kravtsov VC, Zaworotko MJ (2005) Ternary nets formed by self-assembly of triangles, squares, and tetrahedra. Angew Chem Int Ed 44:2877
Agarwal RA, Aijaz A, Ahmad M, Sañudo EC, Xu Q, Bharadwaj PK (2012) Two new coordination polymers with Co(II) and Mn(II): selective gas adsorption and magnetic studies. Cryst Growth Des 12:2999
Ahmad M, Sharma MK, Das R, Poddar P, Bharadwaj PK (2012) Syntheses, crystal structures, and magnetic properties of metal–organic hybrid materials of Co(II) using flexible and rigid nitrogen-based ditopic ligands as spacers. Cryst Growth Des 12:1571
Lisnard L, Mialane P, Dolbecq A, Marrot J, Clemente-Juan JM, Coronado E, Keita B, de Oliveira P, Nadjo L, Secheresse F (2007) Effect of cyanato, azido, carboxylato, and carbonato ligands on the Formation of Cobalt(II) polyoxometalates: characterization, magnetic, and electrochemical studies of multinuclear cobalt clusters. Chem-Eur J 13:3525
Li XJ, Wang XY, Gao S, Cao R (2006) Two three-dimensional metal−organic frameworks containing one-dimensional hydroxyl/carboxylate mixed bridged metal chains: syntheses, crystal structures, and magnetic properties. Inorg Chem 45:1508
The network topology was evaluated by the program “TOPOS-4.0”, see: http://www.topos.ssu.samara.ru; Blatov VA (2006) Multipurpose crystallochemical analysis with the program package. IUCr Comp Comm Newslett 7:4
Blatov VA, Shevchenko AP, Serezhkin VN (2000) TOPOS3.2: a new version of the program package for multipurpose crystal-chemical analysis. J Appl Crystallogr 33:1193
Blatov VA, O’Keeffe M, Proserpio DM (2010) Vertex-, face-, point-, Schläfli-, and Delaney-symbols in nets, polyhedra and tilings: recommended terminology. CrystEngComm 12:44
Mishra R, Ahmad M, Tripathi MR, Butcher RJ (2013) Four novel coordination polymers of transition metals built using a semi rigid oxygen donor ligand: crystal structures, novel topology and emission studies. Polyhedron 50:169
Li M, Xiang JF, Yuan LJ, Wu SM, Chen SP, Sun JT (2006) Syntheses, structures, and photoluminescence of three novel coordination polymers constructed from dimeric d10 metal units. Cryst Growth Des 6:2036
Zang SQ, Su Y, Li YZ, Ni ZP, Meng QJ (2006) One dense and two open chiral metal−organic frameworks: crystal structures and physical properties. Inorg Chem 45:2972
He JH, Yu JH, Zhang YT, Pan QH, Xu RR (2005) Synthesis, structure, and luminescent property of a heterometallic metal-organic framework constructed from rod-shaped secondary building blocks. Inorg Chem 44:9279
Ahmad M, Bharadwaj PK (2013) Synthesis of coordination polymers with d(10) metal ions and a new linear ligand: X-ray structural and luminescence studies. Polyhedron 52:1145
Acknowledgments
PKB gratefully acknowledges the financial support received from the Department of Science and Technology, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Agarwal, R.A., Bharadwaj, P.K. Structural Investigation and Solid State Emission Studies of Co(II), Zn(II) and Cd(II) Coordination Polymers Built with a Bifurcated Ligand. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 84, 251–259 (2014). https://doi.org/10.1007/s40010-014-0138-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-014-0138-4