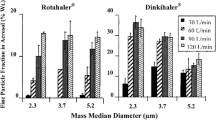
Abstract
Background
There is a growing interest in the use of dry powder inhalers (DPIs) for administering high-dose drugs with low potency to the lungs as carrier-free formulations to treat both local pulmonary and systemic diseases. The main purpose of particle engineering and formulation design in the development of high-dose DPIs without carrier is to reduce the cohesive property of the particles and overcome the large inter-particulate interaction while enhancing powder dispersion and lung delivery upon inhalation and maintaining stability without agglomeration during storage. In particular, the cohesive property of high-dose DPIs can induce several physiological and physicochemical problems that must be improved.
Area covered
This review describes various particle surface modification methods, including sophisticated particle engineering (such as micronization) and formulation techniques, which are utilized to overcome the problems associated with high-dose DPIs.
Expert opinion
Currently, changing the cohesive property of particles through various micronization processes and improving the surface properties of particles through co-processing with limited excipients have been mainly used as efficient methods for particle surface modification. Using these technological principles, novel approaches have been attempted to develop inhalable drug particles, such as high-dose antibiotics and high-value biopharmaceuticals. The research on high-dose DPIs has expanded widely, and consequently, the drugs that received recent regulatory approvals have been successful based on the research results. Thus, it is expected that the next generation of DPIs will be safer and have higher therapeutic efficacy beyond the limitations of traditional high-dose DPIs.




Similar content being viewed by others
References
Abuzar SM, Hyun S-M, Kim J-H, Park HJ, Kim M-S, Park J-S, Hwang S-J (2018) Enhancing the solubility and bioavailability of poorly water-soluble drugs using supercritical antisolvent (SAS) process. Int J Pharm 538:1–13
Adi H, Traini D, Chan H-K, Young PM (2008a) The influence of drug morphology on aerosolisation efficiency of dry powder inhaler formulations. J Pharm Sci 97:2780–2788
Adi H, Young PM, Chan H-K, Stewart P, Agus H, Traini D (2008b) Cospray dried antibiotics for dry powder lung delivery. J Pharm Sci 97:3356–3366
Aguiar-Ricardo A (2017) Building dry powder formulations using supercritical CO2 spray drying. Curr Opin Gr Sustain Chem 5:12–16
Ahsan F, Rivas IP, Khan MA, Suárez AIT (2002) Targeting to macrophages: role of physicochemical properties of particulate carriers—liposomes and microspheres—on the phagocytosis by macrophages. J Control Release 79:29–40
Ali AMA, Abdelrahim MEA (2014) Modeling and optimization of terbutaline emitted from a dry powder inhaler and influence on systemic bioavailability using data mining technology. J Pharm Innov 9:38–47
Ali ME, Lamprecht A (2014) Spray freeze drying for dry powder inhalation of nanoparticles. Eur J Pharm Biopharm 87:510–517
Al-Tabakha MM (2015) Future prospect of insulin inhalation for diabetic patients: the case of Afrezza versus Exubera. J Control Release 215:25–38
Amaro MI, Tajber L, Corrigan OI, Healy AM (2011) Optimisation of spray drying process conditions for sugar nanoporous microparticles (NPMPs) intended for inhalation. Int J Pharm 421:99–109
Amorij J-P, Saluja V, Petersen AH, Hinrichs WL, Huckriede A, Frijlink HW (2007) Pulmonary delivery of an inulin-stabilized influenza subunit vaccine prepared by spray-freeze drying induces systemic, mucosal humoral as well as cell-mediated immune responses in BALB/c mice. Vaccine 25:8707–8717
Aquino R, Prota L, Auriemma G, Santoro A, Mencherini T, Colombo G, Russo P (2012) Dry powder inhalers of gentamicin and leucine: formulation parameters, aerosol performance and in vitro toxicity on CuFi1 cells. Int J Pharm 426:100–107
Aragao-Santiago L, Bohr A, Delaval M, Dalla-Bona CA, Gessler T, Seeger W, Beck-Broichsitter M (2016) Innovative formulations for controlled drug delivery to the lungs and the technical and toxicological challenges to overcome. Curr Pharm Des 22:1147–1160
Arnold MM, Gorman EM, Schieber LJ, Munson EJ, Berkland C (2007) NanoCipro encapsulation in monodisperse large porous PLGA microparticles. J Control Release 121:100–109
Audouy SA, Van Der Schaaf G, Hinrichs WL, Frijlink HW, Wilschut J, Huckriede A (2011) Development of a dried influenza whole inactivated virus vaccine for pulmonary immunization. Vaccine 29:4345–4352
Bae SE, Son JS, Park K, Han DK (2009) Fabrication of covered porous PLGA microspheres using hydrogen peroxide for controlled drug delivery and regenerative medicine. J Control Release 133:37–43
Balani P, Wong S, Ng W, Widjaja E, Tan R, Chan S (2010a) Influence of polymer content on stabilizing milled amorphous salbutamol sulphate. Int J Pharm 391:125–136
Balani PN, Ng WK, Tan RB, Chan SY (2010b) Influence of excipients in comilling on mitigating milling-induced amorphization or structural disorder of crystalline pharmaceutical actives. J Pharm Sci 99:2462–2474
Banaschewski B, Hofmann T (2019) Inhaled antibiotics for mycobacterial lung disease. Pharmaceutics 11:352
Bartus RT, Emerich D, Snodgrass-Belt P, Fu K, Salzberg-Brenhouse H, Lafreniere D, Novak L, Lo E-S, Cooper T, Basile AS (2004) A pulmonary formulation of l-dopa enhances its effectiveness in a rat model of Parkinson’s disease. J Pharmacol Exp Ther 310:828–835
Beach LE (2011) Effect of dry particle coating on the properties of cohesive pharmaceutical powders. Powder Technol. https://doi.org/10.1016/j.powtec.2010.11.028
Begat P, Price R, Harris H, Morton DA, Staniforth JN (2005) The influence of force control agents on the cohesive-adhesive balance in dry powder inhaler formulations. Kona Powder Part J 23:109–121
Begat P, Morton DA, Shur J, Kippax P, Staniforth JN, Price R (2009) The role of force control agents in high-dose dry powder inhaler formulations. J Pharm Sci 98:2770–2783
Ben-Jebria A, Chen D, Eskew ML, Vanbever R, Langer R, Edwards DA (1999) Large porous particles for sustained protection from carbachol-induced bronchoconstriction in guinea pigs. Pharm Res 16:555–561
Boraey MA, Hoe S, Sharif H, Miller DP, Lechuga-Ballesteros D, Vehring R (2013) Improvement of the dispersibility of spray-dried budesonide powders using leucine in an ethanol–water cosolvent system. Powder Technol 236:171–178
Bristow S, Shekunov T, Shekunov BY, York P (2001) Analysis of the supersaturation and precipitation process with supercritical CO2. J Supercrit Fluids 21:257–271
Brodka-Pfeiffer K, Häusler H, Graß P, Langguth P (2003) Conditioning following powder micronization: influence on particle growth of salbutamol sulfate. Drug Dev Ind Pharm 29:1077–1084
Brunaugh A, Smyth H (2018a) Process optimization and particle engineering of micronized drug powders via milling. Drug Deliv Transl Res 8:1740–1750
Brunaugh AD, Smyth HD (2018b) Formulation techniques for high dose dry powders. Int J Pharm 547:489–498
Brunaugh AD, Jan SU, Ferrati S, Smyth HD (2017) Excipient-free pulmonary delivery and macrophage targeting of clofazimine via air jet micronization. Mol Pharm 14:4019–4031
Carvalho TC, Peters JI, Williams Iii RO (2011) Influence of particle size on regional lung deposition–what evidence is there? Int J Pharm 406:1–10
Castellanos A (2005) The relationship between attractive interparticle forces and bulk behaviour in dry and uncharged fine powders. Adv Phys 54:263–376
Chai G, Park H, Yu S, Zhou F, Li J, Xu Q, Zhou QT (2019) Evaluation of co-delivery of colistin and ciprofloxacin in liposomes using an in vitro human lung epithelial cell model. Int J Pharm 569:118616
Chan JGY, Bai X, Traini D (2014) An update on the use of rifapentine for tuberculosis therapy. Expert Opin Drug Deliv 11:421–431
Chang RYK, Chen L, Chen D, Chan H-K (2020) Overcoming challenges for development of amorphous powders for inhalation. Expert Opin Drug Deliv 17:1583–1595
Chen Y, Yang J, Dave RN, Pfeffer R (2008) Fluidization of coated group C powders. AIChE J 54:104–121
Chen L, Okuda T, Lu X-Y, Chan H-K (2016) Amorphous powders for inhalation drug delivery. Adv Drug Deliv Rev 100:102–115
Cheow WS, Ng MLL, Kho K, Hadinoto K (2011) Spray-freeze-drying production of thermally sensitive polymeric nanoparticle aggregates for inhaled drug delivery: effect of freeze-drying adjuvants. Int J Pharm 404:289–300
Chew NY, Chan H-K (2001) Use of solid corrugated particles to enhance powder aerosol performance. Pharm Res 18:1570–1577
Chew NY, Chan H-K (2002) The role of particle properties in pharmaceutical powder inhalation formulations. J Aerosol Med 15:325–330
Chew NY, Tang P, Chan H-K, Raper JA (2005) How much particle surface corrugation is sufficient to improve aerosol performance of powders? Pharm Res 22:148–152
Chow AH, Tong HH, Chattopadhyay P, Shekunov BY (2007) Particle engineering for pulmonary drug delivery. Pharm Res 24:411–437
Cipolla D, Shekunov B, Blanchard J, Hickey A (2014) Lipid-based carriers for pulmonary products: preclinical development and case studies in humans. Adv Drug Deliv Rev 75:53–80
Claus S, Weiler C, Schiewe J, Friess W (2014) How can we bring high drug doses to the lung? Eur J Pharm Biopharm 86:1–6
Cook RO, Pannu RK, Kellaway IW (2005) Novel sustained release microspheres for pulmonary drug delivery. J Control Release 104:79–90
Corcoran T, Venkataramanan R, Hoffman R, George M, Petrov A, Richards T, Zhang S, Choi J, Gao Y, Oakum C (2013) Systemic delivery of atropine sulfate by the microdose dry-powder inhaler. J Aerosol Med Pulm Drug Deliv 26:46–55
Cui Y, Zhang X, Wang W, Huang Z, Zhao Z, Wang G, Cai S, Jing H, Huang Y, Pan X (2018) Moisture-resistant co-spray-dried netilmicin with l-leucine as dry powder inhalation for the treatment of respiratory infections. Pharmaceutics 10:252
D’addio SM, Chan JGY, Kwok PCL, Prud’homme RK, Chan H-K (2012) Constant size, variable density aerosol particles by ultrasonic spray freeze drying. Int J Pharm 427:185–191
Dahmash EZ, Mohammed AR (2015) Functionalised particles using dry powder coating in pharmaceutical drug delivery: promises and challenges. Expert Opin Drug Deliv 12:1867–1879
Dahmash EZ, Mohammed AR (2016) Characterisation and surface-profiling techniques for composite particles produced by dry powder coating in pharmaceutical drug delivery. Drug Discov Today 21:550–561
De Boer AH, Hagedoorn P, Hoppentocht M, Buttini F, Grasmeijer F, Frijlink HW (2017) Dry powder inhalation: past, present and future. Expert Opin Drug Deliv 14:499–512
Djokić M, Kachrimanis K, Solomun L, Djuriš J, Vasiljević D, Ibrić S (2014) A study of jet-milling and spray-drying process for the physicochemical and aerodynamic dispersion properties of amiloride HCl. Powder Technol 262:170–176
Duddu SP, Sisk SA, Walter YH, Tarara TE, Trimble KR, Clark AR, Eldon MA, Elton RC, Pickford M, Hirst PH (2002) Improved lung delivery from a passive dry powder inhaler using an engineered PulmoSphere® powder. Pharm Res 19:689–695
Dufour G, Bigazzi W, Wong N, Boschini F, De Tullio P, Piel G, Cataldo D, Evrard B (2015) Interest of cyclodextrins in spray-dried microparticles formulation for sustained pulmonary delivery of budesonide. Int J Pharm 495:869–878
Dunber CA, Hickey AJ, Holzner P (1998) Dispersion and characterization of pharmaceutical dry powder aerosols. Kona Powder Part J 16:7–45
Duret C, Wauthoz N, Sebti T, Vanderbist F, Amighi K (2012) New inhalation-optimized itraconazole nanoparticle-based dry powders for the treatment of invasive pulmonary aspergillosis. Int J Nanomed 7:5475
Edwards DA, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, Eskew ML, Mintzes J, Deaver D, Lotan N, Langer R (1997) Large porous particles for pulmonary drug delivery. Science 276:1868–1872
Endoh S, Szepvolgyi J, Izumi K, Hotta T, Naito M (2004) Experimental and theoretical analysis of mechanical coating process of particles with the theta composer. Chem Eng Commun 191:1259–1270
Fages J, Lochard H, Letourneau J-J, Sauceau M, Rodier E (2004) Particle generation for pharmaceutical applications using supercritical fluid technology. Powder Technol 141:219–226
Faghihi H, Vatanara A, Najafabadi AR, Ramezani V, Gilani K (2014) The use of amino acids to prepare physically and conformationally stable spray-dried IgG with enhanced aerosol performance. Int J Pharm 466:163–171
Farkas DR, Hindle M, Longest PW (2015) Characterization of a new high-dose dry powder inhaler (DPI) based on a fluidized bed design. Ann Biomed Eng 43:2804–2815
Feeley J, York P, Sumby B, Dicks H (1998a) Determination of surface properties and flow characteristics of salbutamol sulphate, before and after micronisation. Int J Pharm 172:89–96
Feeley J, York P, Sumby B, Dicks H (1998b) Comparison of the surface properties of salbutamol sulphate prepared by micronization and a supercritical fluid technique. J Pharm Pharmacol 50:54–54
Focaroli S, Mah P, Hastedt J, Gitlin I, Oscarson S, Fahy J, Healy A (2019) A design of experiment (DoE) approach to optimise spray drying process conditions for the production of trehalose/leucine formulations with application in pulmonary delivery. Int J Pharm 562:228–240
French DL, Edwards DA, Niven RW (1996) The influence of formulation on emission, deaggregation and deposition of dry powders for inhalation. J Aerosol Sci 27:769–783
Geller DE, Weers J, Heuerding S (2011) Development of an inhaled dry-powder formulation of tobramycin using PulmoSphereTM technology. J Aerosol Med Pulm Drug Deliv 24:175–182
Gera M, Saharan VA, Kataria M, Kukkar V (2010) Mechanical methods for dry particle coating processes and their applications in drug delivery and development. Recent Pat Drug Deliv Formul 4:58–81
Gómez-Gaete C, Fattal E, Silva L, Besnard M, Tsapis N (2008) Dexamethasone acetate encapsulation into trojan particles. J Control Release 128:41–49
Gradon L, Sosnowski TR (2014) Formation of particles for dry powder inhalers. Adv Powder Technol 25:43–55
Han X, Ghoroi C, To D, Chen Y, Davé R (2011) Simultaneous micronization and surface modification for improvement of flow and dissolution of drug particles. Int J Pharm 415:185–195
Han X, Jallo L, To D, Ghoroi C, Davé R (2013) Passivation of high-surface-energy sites of milled ibuprofen crystals via dry coating for reduced cohesion and improved flowability. J Pharm Sci 102:2282–2296
Healy A, Mcdonald B, Tajber L, Corrigan O (2008) Characterisation of excipient-free nanoporous microparticles (NPMPs) of bendroflumethiazide. Eur J Pharm Biopharm 69:1182–1186
Healy AM, Amaro MI, Paluch KJ, Tajber L (2014) Dry powders for oral inhalation free of lactose carrier particles. Adv Drug Deliv Rev 75:32–52
Heng JY, Thielmann F, Williams DR (2006) The effects of milling on the surface properties of form I paracetamol crystals. Pharm Res 23:1918–1927
Hickey A, Gonda I, Irwin W, Fildes F (1990) Effect of hydrophobic coating on the behavior of a hygroscopic aerosol powder in an environment of controlled temperature and relative humidity. J Pharm Sci 79:1009–1014
Hickey AJ, Mansour HM, Telko MJ, Xu Z, Smyth HD, Mulder T, Mclean R, Langridge J, Papadopoulos D (2007) Physical characterization of component particles included in dry powder inhalers. I. Strategy review and static characteristics. J Pharm Sci 96:1282–1301
Hoppentocht M, Hagedoorn P, Frijlink H, De Boer A (2014) Technological and practical challenges of dry powder inhalers and formulations. Adv Drug Deliv Rev 75:18–31
Ibrahim M, Verma R, Garcia-Contreras L (2015) Inhalation drug delivery devices: technology update. Med Devices (Auckland) 8:131
Iida K, Todo H, Okamoto H, Danjo K, Leuenberger H (2005) Preparation of dry powder inhalation with lactose carrier particles surface-coated using a Wurster fluidized bed. Chem Pharm Bull 53:431–434
Ishizaka T, Honda H, Kikuchi Y, Ono K, Katano T, Koishi M (1989) Preparation of drug-diluent hybrid powders by dry processing. J Pharm Pharmacol 41:361–368
Iskandar F, Nandiyanto ABD, Widiyastuti W, Young LS, Okuyama K, Gradon L (2009) Production of morphology-controllable porous hyaluronic acid particles using a spray-drying method. Acta Biomater 5:1027–1034
Islam N, Gladki E (2008) Dry powder inhalers (DPIs)—a review of device reliability and innovation. Int J Pharm 360:1–11
Kaialy W, Nokhodchi A (2015) Particle engineering for improved pulmonary drug delivery through dry powder inhalers. In: Nokhodchi A, Martin GP (eds) Pulmonary drug delivery: advances and challenges. Wiley, Hoboken, pp 171–198
Kazmi A, Lechuga-Ballesteros D, Snyder HE, Ivey J, Vehring R, Speck JH, Dwivedi S (2018) Google Patents
Kim YH, Shing KS (2008) Supercritical fluid-micronized ipratropium bromide for pulmonary drug delivery. Powder Technol 182:25–32
Kim HK, Chung HJ, Park TG (2006a) Biodegradable polymeric microspheres with “open/closed” pores for sustained release of human growth hormone. J Control Release 112:167–174
Kim TK, Yoon JJ, Lee DS, Park TG (2006b) Gas foamed open porous biodegradable polymeric microspheres. Biomaterials 27:152–159
Kim H, Park H, Lee J, Kim TH, Lee ES, Oh KT, Lee KC, Youn YS (2011) Highly porous large poly (lactic-co-glycolic acid) microspheres adsorbed with palmityl-acylated exendin-4 as a long-acting inhalation system for treating diabetes. Biomaterials 32:1685–1693
Knez Z, Weidner E (2003) Particles formation and particle design using supercritical fluids. Curr Opin Solid State Mater Sci 7:353–361
Knez Ž, Škerget M, Hrnčič MK, Čuček D (2014) Supercritical fluid technology for energy and environmental applications. Elsevier, New York, pp 31–67
Konstan MW, Geller DE, Minić P, Brockhaus F, Zhang J, Angyalosi G (2011) Tobramycin inhalation powder for P. Aeruginosa infection in cystic fibrosis: the EVOLVE trial. Pediatr Pulmonol 46:230–238
Koskela J, Morton DA, Stewart PJ, Juppo AM, Lakio S (2018) The effect of mechanical dry coating with magnesium stearate on flowability and compactibility of plastically deforming microcrystalline cellulose powders. Int J Pharm 537:64–72
Koushik K, Kompella UB (2004) Preparation of large porous deslorelin-PLGA microparticles with reduced residual solvent and cellular uptake using a supercritical carbon dioxide process. Pharm Res 21:524–535
Koushik K, Dhanda DS, Cheruvu NP, Kompella UB (2004) Pulmonary delivery of deslorelin: large-porous PLGA particles and HPβCD complexes. Pharm Res 21:1119–1126
Kwon MJ, Bae JH, Kim JJ, Na K, Lee ES (2007) Long acting porous microparticle for pulmonary protein delivery. Int J Pharm 333:5–9
Labiris N, Dolovich M (2003) Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol 56:588–599
Lau M, Young PM, Traini D (2017) A review of co-milling techniques for the production of high dose dry powder inhaler formulation. Drug Dev Ind Pharm 43:1229–1238
Learoyd TP, Burrows JL, French E, Seville PC (2009) Sustained delivery by leucine-modified chitosan spray-dried respirable powders. Int J Pharm 372:97–104
Lechanteur A, Evrard B (2020) Influence of composition and spray-drying process parameters on carrier-free DPI properties and behaviors in the lung: a review. Pharmaceutics 12:55
Lechuga-Ballesteros D, Charan C, Stults CL, Stevenson CL, Miller DP, Vehring R, Tep V, Kuo MC (2008) Trileucine improves aerosol performance and stability of spray-dried powders for inhalation. J Pharm Sci 97:287–302
Lee SH, Teo J, Heng D, Ng WK, Zhao Y, Tan RB (2016) Tailored antibiotic combination powders for inhaled rotational antibiotic therapy. J Pharm Sci 105:1501–1512
Leena MM, Antoniraj MG, Moses J, Anandharamakrishnan C (2020) Three fluid nozzle spray drying for co-encapsulation and controlled release of curcumin and resveratrol. J Drug Deliv Sci Technol 57:101678
Lefebvre G, Galet L, Chamayou A (2011) Dry coating of talc particles with fumed silica: influence of the silica concentration on the wettability and dispersibility of the composite particles. Powder Technol 208:372–377
Leng D, Thanki K, Foged C, Yang M (2018) Formulating inhalable dry powders using two-fluid and three-fluid nozzle spray drying. Pharm Res 35:1–11
Leng D, Kissi EO, Löbmann K, Thanki K, Fattal E, Rades T, Foged C, Yang M (2019) Design of inhalable solid dosage forms of budesonide and theophylline for pulmonary combination therapy. AAPS PharmSciTech 20:1–11
Li L, Sun S, Parumasivam T, Denman JA, Gengenbach T, Tang P, Mao S, Chan H-K (2016) l-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. Eur J Pharm Biopharm 102:132–141
Li L, Leung SSY, Gengenbach T, Yu J, Gao GF, Tang P, Zhou QT, Chan H-K (2017) Investigation of l-leucine in reducing the moisture-induced deterioration of spray-dried salbutamol sulfate power for inhalation. Int J Pharm 530:30–39
Lin Y-W, Wong J, Qu L, Chan H-K, Zhou QT (2015) Powder production and particle engineering for dry powder inhaler formulations. Curr Pharm Des 21:3902–3916
Littringer EM, Mescher A, Eckhard S, Schröttner H, Langes C, Fries M, Griesser U, Walzel P, Urbanetz NA (2012) Spray drying of mannitol as a drug carrier—the impact of process parameters on product properties. Drying Technol 30:114–124
Liu Q, Guan J, Sun Z, Shen X, Li L, Jin L, Mao S (2019) Influence of stabilizer type and concentration on the lung deposition and retention of resveratrol nanosuspension-in-microparticles. Int J Pharm 569:118562
Lu W, Rades T, Rantanen J, Yang M (2019) Inhalable co-amorphous budesonide-arginine dry powders prepared by spray drying. Int J Pharm 565:1–8
Luinstra M, Grasmeijer F, Hagedoorn P, Moes JR, Frijlink HW, De Boer AH (2015) A levodopa dry powder inhaler for the treatment of Parkinson’s disease patients in off periods. Eur J Pharm Biopharm 97:22–29
Luner PE, Zhang Y, Abramov YA, Carvajal MT (2012) Evaluation of milling method on the surface energetics of molecular crystals using inverse gas chromatography. Cryst Growth Des 12:5271–5282
Maa Y-F, Costantino HR, Nguyen P-A, Hsu CC (1997) The effect of operating and formulation variables on the morphology of spray-dried protein particles. Pharm Dev Technol 2:213–223
Maa Y-F, Nguyen P-A, Sweeney T, Shire SJ, Hsu CC (1999) Protein inhalation powders: spray drying vs spray freeze drying. Pharm Res 16:249–254
Malamatari M, Somavarapu S, Bloxham M, Buckton G (2015) Nanoparticle agglomerates of indomethacin: the role of poloxamers and matrix former on their dissolution and aerosolisation efficiency. Int J Pharm 495:516–526
Malamatari M, Somavarapu S, Kachrimanis K, Bloxham M, Taylor KM, Buckton G (2016a) Preparation of theophylline inhalable microcomposite particles by wet milling and spray drying: the influence of mannitol as a co-milling agent. Int J Pharm 514:200–211
Malamatari M, Somavarapu S, Taylor KM, Buckton G (2016b) Solidification of nanosuspensions for the production of solid oral dosage forms and inhalable dry powders. Expert Opin Drug Deliv 13:435–450
Malamatari M, Somavarapu S, Kachrimanis K, Buckton G, Taylor KM (2017) Preparation of respirable nanoparticle agglomerates of the low melting and ductile drug ibuprofen: impact of formulation parameters. Powder Technol 308:123–134
Malamatari M, Charisi A, Malamataris S, Kachrimanis K, Nikolakakis I (2020) Spray drying for the preparation of nanoparticle-based drug formulations as dry powders for inhalation. Processes 8:788
Malcolmson RJ, Embleton JK (1998) Dry powder formulations for pulmonary delivery. Pharm Sci Technol Today 1:394–398
Mangal S, Park H, Zeng L, Heidi HY, Lin Y-W, Velkov T, Denman JA, Zemlyanov D, Li J, Zhou QT (2018) Composite particle formulations of colistin and meropenem with improved in-vitro bacterial killing and aerosolization for inhalation. Int J Pharm 548:443–453
Mangal S, Park H, Nour R, Shetty N, Cavallaro A, Zemlyanov D, Thalberg K, Puri V, Nicholas M, Narang AS (2019a) Correlations between surface composition and aerosolization of jet-milled dry powder inhaler formulations with pharmaceutical lubricants. Int J Pharm 568:118504
Mangal S, Huang J, Shetty N, Park H, Lin Y-W, Heidi HY, Zemlyanov D, Velkov T, Li J, Zhou QT (2019b) Effects of the antibiotic component on in-vitro bacterial killing, physico-chemical properties, aerosolization and dissolution of a ternary-combinational inhalation powder formulation of antibiotics for pan-drug resistant gram-negative lung infections. Int J Pharm 561:102–113
Mangal S, Xu R, Park H, Zemlyanov D, Shetty N, Lin Y-W, Morton D, Chan H-K, Li J, Zhou QT (2019c) Understanding the impacts of surface compositions on the in-vitro dissolution and aerosolization of co-spray-dried composite powder formulations for inhalation. Pharm Res 36:6
Maretti E, Rossi T, Bondi M, Croce MA, Hanuskova M, Leo E, Sacchetti F, Iannuccelli V (2014) Inhaled solid lipid microparticles to target alveolar macrophages for tuberculosis. Int J Pharm 462:74–82
Martin A, Cocero MJ (2008) Micronization processes with supercritical fluids: fundamentals and mechanisms. Adv Drug Deliv Rev 60:339–350
Mawson S, Kanakia S, Johnston KP (1997) Coaxial nozzle for control of particle morphology in precipitation with a compressed fluid antisolvent. J Appl Polym Sci 64:2105–2118
Mckeage K (2013) Tobramycin inhalation powder: a review of its use in the treatment of chronic Pseudomonas aeruginosa infection in patients with cystic fibrosis. Drugs 73:1815–1827
Mehta P (2018) Imagine the superiority of dry powder inhalers from carrier engineering. J Drug Deliv. https://doi.org/10.1155/2018/5635010
Mehta PP, Pawar AP, Mahadik KR, Kadam SS, Dhapte-Pawar V (2020) Dry powder coating techniques and role of force controlling agents in aerosol. In: Inamuddin I, Boddula R, Ahamed MI, Asiri AM (eds) Polymer coatings: technology and applications. Wiley, Hoboken, pp 41–74
Meng X, Zu Y, Zhao X, Li Q, Jiang S, Sang M (2012) Characterization and pharmacokinetics of coenzyme Q10 nanoparticles prepared by a rapid expansion of supercritical solution process. Pharmazie 67:161
Miller DP, Tan T, Nakamura J, Malcolmson RJ, Tarara TE, Weers JG (2017) Physical characterization of tobramycin inhalation powder: II. State diagram of an amorphous engineered particle formulation. Mol Pharm 14:1950–1960
Mizoe T, Ozeki T, Okada H (2008) Application of a four-fluid nozzle spray drier to prepare inhalable rifampicin-containing mannitol microparticles. AAPS PharmSciTech 9:755–761
Monteith M, Thomas M (2006) Google Patents
Murugappan S, Patil HP, Kanojia G, Ter Veer W, Meijerhof T, Frijlink HW, Huckriede A, Hinrichs WL (2013) Physical and immunogenic stability of spray freeze-dried influenza vaccine powder for pulmonary delivery: comparison of inulin, dextran, or a mixture of dextran and trehalose as protectants. Eur J Pharm Biopharm 85:716–725
Muttil P, Prego C, Garcia-Contreras L, Pulliam B, Fallon JK, Wang C, Hickey AJ, Edwards D (2010) Immunization of guinea pigs with novel hepatitis B antigen as nanoparticle aggregate powders administered by the pulmonary route. AAPS J 12:330–337
Nakach M, Authelin J-R, Chamayou A, Dodds J (2004) Comparison of various milling technologies for grinding pharmaceutical powders. Int J Miner Process 74:S173–S181
Newhouse MT, Hirst PH, Duddu SP, Walter YH, Tarara TE, Clark AR, Weers JG (2003) Inhalation of a dry powder tobramycin PulmoSphereTM formulation in healthy volunteers. Chest 124:360–366
Ng WK, Kwek JW, Tan RB (2008) Anomalous particle size shift during post-milling storage. Pharm Res 25:1175–1185
Ngan CL, Asmawi AA (2018) Lipid-based pulmonary delivery system: a review and future considerations of formulation strategies and limitations. Drug Deliv Transl Res 8:1527–1544
NíÕgáin O, Tajber L, Corrigan OI, Healy AM (2012) Spray drying from organic solvents to prepare nanoporous/nanoparticulate microparticles of protein: excipient composites designed for oral inhalation. J Pharm Pharmacol 64:1275–1290
Niwa T, Mizutani D, Danjo K (2012) Spray freeze-dried porous microparticles of a poorly water-soluble drug for respiratory delivery. Chem Pharm Bull 60:870–876
Nolan LM, Tajber L, Mcdonald BF, Barham AS, Corrigan OI, Healy AM (2009) Excipient-free nanoporous microparticles of budesonide for pulmonary delivery. Eur J Pharm Sci 37:593–602
Nolan LM, Li J, Tajber L, Corrigan OI, Healy AM (2011) Particle engineering of materials for oral inhalation by dry powder inhalers. II—sodium cromoglicate. Int J Pharm 405:36–46
Õgáin ON, Li J, Tajber L, Corrigan OI, Healy AM (2011) Particle engineering of materials for oral inhalation by dry powder inhalers. I—particles of sugar excipients (trehalose and raffinose) for protein delivery. Int J Pharm 405:23–35
Oh YJ, Lee J, Seo JY, Rhim T, Kim S-H, Yoon HJ, Lee KY (2011) Preparation of budesonide-loaded porous PLGA microparticles and their therapeutic efficacy in a murine asthma model. J Control Release 150:56–62
Parsian AR, Vatanara A, Rahmati MR, Gilani K, Khosravi KM, Najafabadi AR (2014) Inhalable budesonide porous microparticles tailored by spray freeze drying technique. Powder Technol 260:36–41
Pasquali I, Bettini R, Giordano F (2006) Solid-state chemistry and particle engineering with supercritical fluids in pharmaceutics. Eur J Pharm Sci 27:299–310
Pasquali I, Bettini R, Giordano F (2008) Supercritical fluid technologies: an innovative approach for manipulating the solid-state of pharmaceuticals. Adv Drug Deliv Rev 60:399–410
Patel B, Gupta V, Ahsan F (2012) PEG–PLGA based large porous particles for pulmonary delivery of a highly soluble drug, low molecular weight heparin. J Control Release 162:310–320
Patel B, Gupta N, Ahsan F (2015) Particle engineering to enhance or lessen particle uptake by alveolar macrophages and to influence the therapeutic outcome. Eur J Pharm Biopharm 89:163–174
Patil-Gadhe AA, Kyadarkunte AY, Pereira M, Jejurikar G, Patole MS, Risbud A, Pokharkar VB (2014) Rifapentine-proliposomes for inhalation: in vitro and in vivo toxicity. Toxicol Int 21:275
Payne NI, Ambrose CV, Timmins P, Ward MD, Ridgway F (1986) Proliposomes: a novel solution to an old problem. J Pharm Sci 75:325–329
Peng T, Zhang X, Huang Y, Zhao Z, Liao Q, Xu J, Huang Z, Zhang J, Wu C-Y, Pan X (2017) Nanoporous mannitol carrier prepared by non-organic solvent spray drying technique to enhance the aerosolization performance for dry powder inhalation. Sci Rep 7:46517
Pfeffer R, Dave RN, Wei D, Ramlakhan M (2001) Synthesis of engineered particulates with tailored properties using dry particle coating. Powder Technol 117:40–67
Phillips EM, Stella VJ (1993) Rapid expansion from supercritical solutions: application to pharmaceutical processes. Int J Pharm 94:1–10
Pilcer G, Sebti T, Amighi K (2006) Formulation and characterization of lipid-coated tobramycin particles for dry powder inhalation. Pharm Res 23:931–940
Pilcer G, De Bueger V, Traina K, Traore H, Sebti T, Vanderbist F, Amighi K (2013a) Carrier-free combination for dry powder inhalation of antibiotics in the treatment of lung infections in cystic fibrosis. Int J Pharm 451:112–120
Pilcer G, Rosière R, Traina K, Sebti T, Vanderbist F, Amighi K (2013b) New co-spray-dried tobramycin nanoparticles-clarithromycin inhaled powder systems for lung infection therapy in cystic fibrosis patients. J Pharm Sci 102:1836–1846
Pomázi A, Buttini F, Ambrus R, Colombo P, Szabó-Révész P (2013) Effect of polymers for aerolization properties of mannitol-based microcomposites containing meloxicam. Eur Polymer J 49:2518–2527
Prosapio V, De Marco I, Reverchon E (2018) Supercritical antisolvent coprecipitation mechanisms. J Supercrit Fluids 138:247–258
Qian L, Zhang H (2011) Controlled freezing and freeze drying: a versatile route for porous and micro-/nano-structured materials. J Chem Technol Biotechnol 86:172–184
Qu L, Av Morton D (2015) Particle engineering via mechanical dry coating in the design of pharmaceutical solid dosage forms. Curr Pharm Des 21:5802–5814
Quevedo J, Pfeffer R, Shen Y, Dave R, Nakamura H, Watano S (2006) Fluidization of nanoagglomerates in a rotating fluidized bed. AIChE J 52:2401–2412
Rachelefsky GS, Liao Y, Faruqi R (2007) Impact of inhaled corticosteroid-induced oropharyngeal adverse events: results from a meta-analysis. Ann Allergy Asthma Immunol 98:225–238
Rad RT, Dadashzadeh S, Vatanara A, Alavi S, Ghasemian E, Mortazavi SA (2019) Tadalafil nanocomposites as a dry powder formulation for inhalation, a new strategy for pulmonary arterial hypertension treatment. Eur J Pharm Sci 133:275–286
Ramezani V, Vatanara A, Seyedabadi M, Nabi Meibodi M, Fanaei H (2017) Application of cyclodextrins in antibody microparticles: potentials for antibody protection in spray drying. Drug Dev Ind Pharm 43:1103–1111
Rasenack N, Müller BW (2004) Micron-size drug particles: common and novel micronization techniques. Pharm Dev Technol 9:1–13
Rattanupatam T, Srichana T (2014) Budesonide dry powder for inhalation: effects of leucine and mannitol on the efficiency of delivery. Drug Delivery 21:397–405
Raula J, Lähde A, Kauppinen EI (2009) Aerosolization behavior of carrier-free l-leucine coated salbutamol sulphate powders. Int J Pharm 365:18–25
Raula J, Thielmann F, Naderi M, Lehto V-P, Kauppinen EI (2010) Investigations on particle surface characteristics vs. dispersion behaviour of l-leucine coated carrier-free inhalable powders. Int J Pharm 385:79–85
Rehman M, Shekunov BY, York P, Lechuga-Ballesteros D, Miller DP, Tan T, Colthorpe P (2004) Optimisation of powders for pulmonary delivery using supercritical fluid technology. Eur J Pharm Sci 22:1–17
Reverchon E, Della Porta G (1999) Production of antibiotic micro-and nano-particles by supercritical antisolvent precipitation. Powder Technol 106:23–29
Reverchon E, Della Porta G, Pallado P (2001) Supercritical antisolvent precipitation of salbutamol microparticles. Powder Technol 114:17–22
Reverchon E, Porta GD, Spada A, Antonacci A (2004) Griseofulvin micronization and dissolution rate improvement by supercritical assisted atomization. J Pharm Pharmacol 56:1379–1387
Rojanarat W, Changsan N, Tawithong E, Pinsuwan S, Chan H-K, Srichana T (2011) Isoniazid proliposome powders for inhalation—preparation, characterization and cell culture studies. Int J Mol Sci 12:4414–4434
Rosenstock J, Muchmore D, Swanson D, Schmitke J (2007) AIR® inhaled insulin system: a novel insulin-delivery system for patients with diabetes. Expert Rev Med Devices 4:683–692
Rowe R (1988) Interaction of lubricants with microcrystalline cellulose and anhydrous lactose—a solubility parameter approach. Int J Pharm 41:223–226
Ruge CA, Bohr A, Beck-Broichsitter M, Nicolas V, Tsapis N, Fattal E (2016) Disintegration of nano-embedded microparticles after deposition on mucus: a mechanistic study. Colloids Surf B 139:219–227
Scherließ R, Etschmann C (2018) DPI formulations for high dose applications—challenges and opportunities. Int J Pharm 548:49–53
Schuster A, Haliburn C, Döring G, Goldman MH, Freedom Study Group (2013) Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: a randomised study. Thorax 68:344–350
Seville PC, Learoyd T, Li H-Y, Williamson I, Birchall JC (2007) Amino acid-modified spray-dried powders with enhanced aerosolisation properties for pulmonary drug delivery. Powder Technol 178:40–50
Shah UV, Olusanmi D, Narang AS, Hussain MA, Tobyn MJ, Hinder SJ, Heng JY (2015) Decoupling the contribution of surface energy and surface area on the cohesion of pharmaceutical powders. Pharm Res 32:248–259
Shah UV, Karde V, Ghoroi C, Heng JY (2017) Influence of particle properties on powder bulk behaviour and processability. Int J Pharm 518:138–154
Shekunov BY, Baldyga J, York P (2001) Particle formation by mixing with supercritical antisolvent at high Reynolds numbers. Chem Eng Sci 56:2421–2433
Shekunov BY, Feeley JC, Chow AH, Tong HH, York P (2003) Aerosolisation behaviour of micronised and supercritically-processed powders. J Aerosol Sci 34:553–568
Shetty N, Ahn P, Park H, Bhujbal S, Zemlyanov D, Cavallaro A, Mangal S, Li J, Zhou QT (2018a) Improved physical stability and aerosolization of inhalable amorphous ciprofloxacin powder formulations by incorporating synergistic colistin. Mol Pharm 15:4004–4020
Shetty N, Park H, Zemlyanov D, Mangal S, Bhujbal S, Zhou QT (2018b) Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation. Int J Pharm 544:222–234
Shetty N, Zeng L, Mangal S, Nie H, Rowles MR, Guo R, Han Y, Park JH, Zhou QT (2018c) Effects of moisture-induced crystallization on the aerosol performance of spray dried amorphous ciprofloxacin powder formulations. Pharm Res 35:1–13
Shetty N, Cipolla D, Park H, Zhou QT (2020) Physical stability of dry powder inhaler formulations. Expert Opin Drug Deliv 17:77–96
Shoyele SA, Cawthorne S (2006) Particle engineering techniques for inhaled biopharmaceuticals. Adv Drug Deliv Rev 58:1009–1029
Shur J, Nevell TG, Ewen RJ, Price R, Smith A, Barbu E, Conway JH, Carroll MP, Shute JK, Smith JR (2008) Cospray-dried unfractionated heparin with l-leucine as a dry powder inhaler mucolytic for cystic fibrosis therapy. J Pharm Sci 97:4857–4868
Shur J, Price R, Lewis D, Young PM, Woollam G, Singh D, Edge S (2016) From single excipients to dual excipient platforms in dry powder inhaler products. Int J Pharm 514:374–383
Sibum I, Hagedoorn P, De Boer AH, Frijlink HW, Grasmeijer F (2018) Challenges for pulmonary delivery of high powder doses. Int J Pharm 548:325–336
Sibum I, Hagedoorn P, Kluitman MP, Kloezen M, Frijlink HW, Grasmeijer F (2020) Dispersibility and storage stability optimization of high dose isoniazid dry powder inhalation formulations with l-leucine or trileucine. Pharmaceutics 12:24
Sievers R, Huang E, Villa J, Engling G, Brauer P (2003a) Micronization of water-soluble or alcohol-soluble pharmaceuticals and model compounds with a low-temperature Bubble Dryer®. J Supercrit Fluids 26:9–16
Sievers RE, Sellers SP, Carpenter JF (2003b) Google Patents
Šimková K, Joost B, Imanidis G (2020) Production of fast-dissolving low-density powders for improved lung deposition by spray drying of a nanosuspension. Eur J Pharm Biopharm 146:19–31
Simon A, Amaro MI, Cabral LM, Healy AM, De Sousa VP (2016) Development of a novel dry powder inhalation formulation for the delivery of rivastigmine hydrogen tartrate. Int J Pharm 501:124–138
Sinha B, Müller RH, Möschwitzer JP (2013) Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm 453:126–141
Smith IJ, Parry-Billings M (2003) The inhalers of the future? A review of dry powder devices on the market today. Pulm Pharmacol Ther 16:79–95
Stank K, Steckel H (2013) Physico-chemical characterisation of surface modified particles for inhalation. Int J Pharm 448:9–18
Stass H, Nagelschmitz J, Willmann S, Delesen H, Gupta A, Baumann S (2013) Inhalation of a dry powder ciprofloxacin formulation in healthy subjects: a phase I study. Clin Drug Investig 33:419–427
Steckel H, Bolzen N (2004) Alternative sugars as potential carriers for dry powder inhalations. Int J Pharm 270:297–306
Steckel H, Brandes HG (2004) A novel spray-drying technique to produce low density particles for pulmonary delivery. Int J Pharm 278:187–195
Steckel H, Rasenack N, Villax P, Müller BW (2003) In vitro characterization of jet-milled and in-situ-micronized fluticasone-17-propionate. Int J Pharm 258:65–75
Steckel H, Pichert L, Müller BW (2004) Influence of process parameters in the ASES process on particle properties of budesonide for pulmonary delivery. Eur J Pharm Biopharm 57:507–512
Steckel H, Markefka P, Kammelar R (2006) Effect of milling and sieving on functionality of dry powder inhalation products. Int J Pharm 309:51–59
Storm G, Crommelin DJ (1998) Liposomes: quo vadis? Pharm Sci Technol Today 1:19–31
Sun Y (2015) Supercritical fluid particle design of DPI formulations. Curr Pharm Des 21:2516–2542
Sun Y (2016) Carrier free inhaled dry powder of budesonide tailored by supercritical fluid particle design. Powder Technol 304:248–260
Sun R, Lu Y, Chen K (2009) Preparation and characterization of hollow hydroxyapatite microspheres by spray drying method. Mater Sci Eng C 29:1088–1092
Sung JC, Padilla DJ, Garcia-Contreras L, Verberkmoes JL, Durbin D, Peloquin CA, Elbert KJ, Hickey AJ, Edwards DA (2009) Formulation and pharmacokinetics of self-assembled rifampicin nanoparticle systems for pulmonary delivery. Pharm Res 26:1847–1855
Tay T, Das S, Stewart P (2010) Magnesium stearate increases salbutamol sulphate dispersion: what is the mechanism? Int J Pharm 383:62–69
Taylor K, Pancholi K, Wong D (1999) In-vitro evaluation of dry powder inhaler formulations of micronized and milled nedocromil sodium. Pharm Pharmacol Commun 5:255–257
Telko MJ, Hickey AJ (2005) Dry powder inhaler formulation. Respir Care 50:1209–1227
Teng S, Wang P, Zhu L, Young M-W, Gogos CG (2009) Experimental and numerical analysis of a lab-scale fluid energy mill. Powder Technol 195:31–39
Tewes F, Paluch KJ, Tajber L, Gulati K, Kalantri D, Ehrhardt C, Healy AM (2013) Steroid/mucokinetic hybrid nanoporous microparticles for pulmonary drug delivery. Eur J Pharm Biopharm 85:604–613
Thai A, Xiao J, Ammit A, Rohanizadeh R (2010) Development of inhalable formulations of anti-inflammatory drugs to potentially treat smoke inhalation injury in burn victims. Int J Pharm 389:41–52
Tomoda K, Ohkoshi T, Kawai Y, Nishiwaki M, Nakajima T, Makino K (2008a) Preparation and properties of inhalable nanocomposite particles: effects of the temperature at a spray-dryer inlet upon the properties of particles. Colloids Surf B 61:138–144
Tomoda K, Ohkoshi T, Nakajima T, Makino K (2008b) Preparation and properties of inhalable nanocomposite particles: effects of the size, weight ratio of the primary nanoparticles in nanocomposite particles and temperature at a spray-dryer inlet upon properties of nanocomposite particles. Colloids Surf B 64:70–76
Tong HH, Chow AH (2006) Control of physical forms of drug particles for pulmonary delivery by spray drying and supercritical fluid processing. Kona Powder Part J 24:27–40
Tong HH, Shekunov BY, York P, Chow AH (2001) Characterization of two polymorphs of salmeterol xinafoate crystallized from supercritical fluids. Pharm Res 18:852–858
Toropainen T, Velaga S, Heikkilä T, Matilainen L, Jarho P, Carlfors J, Lehto VP, Järvinen T, Järvinen K (2006) Preparation of budesonide/γ-cyclodextrin complexes in supercritical fluids with a novel SEDS method. J Pharm Sci 95:2235–2245
Traini D, Scalia S, Adi H, Marangoni E, Young PM (2012) Polymer coating of carrier excipients modify aerosol performance of adhered drugs used in dry powder inhalation therapy. Int J Pharm 438:150–159
Tsai W-C, Rizvi SS (2016) Liposomal microencapsulation using the conventional methods and novel supercritical fluid processes. Trends Food Sci Technol 55:61–71
Tsapis N, Bennett D, Jackson B, Weitz DA, Edwards D (2002) Trojan particles: large porous carriers of nanoparticles for drug delivery. Proc Natl Acad Sci 99:12001–12005
Ungaro F, D’angelo I, Miro A, La Rotonda MI, Quaglia F (2012a) Engineered PLGA nano-and micro-carriers for pulmonary delivery: challenges and promises. J Pharm Pharmacol 64:1217–1235
Ungaro F, D’angelo I, Coletta C, Di Villa Bianca RDE, Sorrentino R, Perfetto B, Tufano MA, Miro A, La Rotonda MI, Quaglia F (2012b) Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J Controll Release 157:149–159
Vanbever R, Mintzes JD, Wang J, Nice J, Chen D, Batycky R, Langer R, Edwards DA (1999) Formulation and physical characterization of large porous particles for inhalation. Pharm Res 16:1735–1742
Vandevanter DR, Geller DE (2011) Tobramycin administered by the TOBI® Podhaler® for persons with cystic fibrosis: a review. Med Devices (Auckland) 4:179
Vehring R (2008) Pharmaceutical particle engineering via spray drying. Pharm Res 25:999–1022
Velaga SP, Berger R, Carlfors J (2002) Supercritical fluids crystallization of budesonide and flunisolide. Pharm Res 19:1564–1571
Verma S, Gokhale R, Burgess DJ (2009) A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int J Pharm 380:216–222
Vidgren M, Vidgren P, Paronen T (1987) Comparison of physical and inhalation properties of spray-dried and mechanically micronized disodium cromoglycate. Int J Pharm 35:139–144
Visser J (1989) Van der Waals and other cohesive forces affecting powder fluidization. Powder Technol 58:1–10
Vozone CM, Marques HMC (2002) Complexation of budesonide in cyclodextrins and particle aerodynamic characterization of the complex solid form for dry powder inhalation. J Incl Phenom Macrocycl Chem 44:111–116
Wang Y, Kho K, Cheow WS, Hadinoto K (2012) A comparison between spray drying and spray freeze drying for dry powder inhaler formulation of drug-loaded lipid–polymer hybrid nanoparticles. Int J Pharm 424:98–106
Ward GH, Schultz RK (1995) Process-induced crystallinity changes in albuterol sulfate and its effect on powder physical stability. Pharm Res 12:773–779
Warren E, Morgan K, Toward TJ, Schwenkglenks M, Leadbetter J (2019) Cost effectiveness of inhaled mannitol (Bronchitol®) in patients with cystic fibrosis. Pharmacoeconomics 37:435–446
Weers J (2015) Inhaled antimicrobial therapy–barriers to effective treatment. Adv Drug Deliv Rev 85:24–43
Weers JG, Miller DP (2015) Formulation design of dry powders for inhalation. J Pharm Sci 104:3259–3288
Weers J, Tarara T (2014) The PulmoSphereTM platform for pulmonary drug delivery. Ther Deliv 5:277–295
Weers JG, Tarara TE, Clark AR (2007) Design of fine particles for pulmonary drug delivery. Expert Opin Drug Deliv 4:297–313
Weiler C, Egen M, Trunk M, Langguth P (2010) Force control and powder dispersibility of spray dried particles for inhalation. J Pharm Sci 99:303–316
Wong J, Kwok PCL, Noakes T, Fathi A, Dehghani F, Chan H-K (2014) Effect of crystallinity on electrostatic charging in dry powder inhaler formulations. Pharm Res 31:1656–1664
Wu X, Li X, Mansour HM (2010) Surface analytical techniques in solid-state particle characterization for predicting performance in dry powder inhalers. Kona Powder Part J 28:3–19
Xia Y, Su Y, Wang Q, Yang C, Tang B, Zhang Y, Tu J, Shen Y (2019) Preparation, characterization, and pharmacodynamics of insulin-loaded fumaryl diketopiperazine microparticle dry powder inhalation. Drug Delivery 26:650–660
Xu Z, Mansour HM, Hickey AJ (2011) Particle interactions in dry powder inhaler unit processes: a review. J Adhes Sci Technol 25:451–482
Yamamoto H, Hoshina W, Kurashima H, Takeuchi H, Kawashima Y, Yokoyama T, Tsujimoto H (2007) Engineering of poly (DL-lactic-co-glycolic acid) nanocomposite particles for dry powder inhalation dosage forms of insulin with the spray-fluidized bed granulating system. Adv Powder Technol 18:215–228
Yamasaki K, Kwok PCL, Fukushige K, Prud’homme RK, Chan H-K (2011) Enhanced dissolution of inhalable cyclosporine nano-matrix particles with mannitol as matrix former. Int J Pharm 420:34–42
Yang Y, Bajaj N, Xu P, Ohn K, Tsifansky MD, Yeo Y (2009) Development of highly porous large PLGA microparticles for pulmonary drug delivery. Biomaterials 30:1947–1953
Yang X-B, Wang X-B, Wei SP, Xi R-G, Wang Y-N, Liu D, Shi Y, Jiang S (2011) Optimization and characterization of dry powder of fanhuncaoin for inhalation based on selection of excipients. Chem Pharm Bull 59:929–937
Yang MY, Chan JGY, Chan H-K (2014) Pulmonary drug delivery by powder aerosols. J Control Release 193:228–240
Yang X-F, Xu Y, Qu D-S, Li H-Y (2015) The influence of amino acids on aztreonam spray-dried powders for inhalation. Asian J Pharm Sci 10:541–548
Yazdi AK, Smyth HD (2016) Carrier-free high-dose dry powder inhaler formulation of ibuprofen: physicochemical characterization and in vitro aerodynamic performance. Int J Pharm 511:403–414
Ye T, Sun S, Sugianto TD, Tang P, Parumasivam T, Chang YK, Astudillo A, Wang S, Chan H-K (2018) Novel combination proliposomes containing tobramycin and clarithromycin effective against Pseudomonas aeruginosa biofilms. Int J Pharm 552:130–138
Yu Z, Garcia AS, Johnston KP, Williams RO (2004) Spray freezing into liquid nitrogen for highly stable protein nanostructured microparticles. Eur J Pharm Biopharm 58:529–537
Yu J, Chan H-K, Gengenbach T, Denman JA (2017) Protection of hydrophobic amino acids against moisture-induced deterioration in the aerosolization performance of highly hygroscopic spray-dried powders. Eur J Pharm Biopharm 119:224–234
Yu J, Romeo M-C, Cavallaro AA, Chan H-K (2018) Protective effect of sodium stearate on the moisture-induced deterioration of hygroscopic spray-dried powders. Int J Pharm 541:11–18
Zeng XM, Martin GP, Marriott C, Pritchard J (2000) The influence of carrier morphology on drug delivery by dry powder inhalers. Int J Pharm 200:93–106
Zhang Y, Trissel LA (2006) Physical and chemical stability of pemetrexed in infusion solutions. Ann Pharmacother 40:1082–1085
Zhang Y, Wang X, Lin X, Liu X, Tian B, Tang X (2010) High azithromycin loading powders for inhalation and their in vivo evaluation in rats. Int J Pharm 395:205–214
Zhao Z, Huang Z, Zhang X, Huang Y, Cui Y, Ma C, Wang G, Freeman T, Lu X-Y, Pan X (2018) Low density, good flowability cyclodextrin-raffinose binary carrier for dry powder inhaler: anti-hygroscopicity and aerosolization performance enhancement. Expert Opin Drug Deliv 15:443–457
Zhou QT, Morton DA (2012) Drug-lactose binding aspects in adhesive mixtures: controlling performance in dry powder inhaler formulations by altering lactose carrier surfaces. Adv Drug Deliv Rev 64:275–284
Zhou Q, Armstrong B, Larson I, Stewart PJ, Morton DA (2010a) Improving powder flow properties of a cohesive lactose monohydrate powder by intensive mechanical dry coating. J Pharm Sci 99:969–981
Zhou QT, Qu L, Larson I, Stewart PJ, Morton DA (2010b) Improving aerosolization of drug powders by reducing powder intrinsic cohesion via a mechanical dry coating approach. Int J Pharm 394:50–59
Zhou QT, Qu L, Gengenbach T, Larson I, Stewart PJ, Morton DA (2013) Effect of surface coating with magnesium stearate via mechanical dry powder coating approach on the aerosol performance of micronized drug powders from dry powder inhalers. AAPS PharmSciTech 14:38–44
Zhou QT, Gengenbach T, Denman JA, Heidi HY, Li J, Chan HK (2014) Synergistic antibiotic combination powders of colistin and rifampicin provide high aerosolization efficiency and moisture protection. AAPS J 16:37–47
Zhou QT, Loh ZH, Yu J, Sun S-P, Gengenbach T, Denman JA, Li J, Chan H-K (2016) How much surface coating of hydrophobic azithromycin is sufficient to prevent moisture-induced decrease in aerosolisation of hygroscopic amorphous colistin powder? AAPS J 18:1213–1224
Zhu K, Tan RB, Ng WK, Shen S, Zhou Q, Heng PW (2008) Analysis of the influence of relative humidity on the moisture sorption of particles and the aerosolization process in a dry powder inhaler. J Aerosol Sci 39:510–524
Zijlstra GS, Hinrichs WL, De Boer AH, Frijlink HW (2004) The role of particle engineering in relation to formulation and de-agglomeration principle in the development of a dry powder formulation for inhalation of cetrorelix. Eur J Pharm Sci 23:139–149
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (Grant No. 2020R1A2C4002166). This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. 2020R1I1A1A01074378). I would like to thank Sebin Kim for helping search for literature with this review paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors (H. Park, E.S. Ha, and M.S. Kim) declare that they have no conflict of interest.
Research involving human and animal rights
This article does not contain any studies with human and animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, H., Ha, ES. & Kim, MS. Surface modification strategies for high-dose dry powder inhalers. J. Pharm. Investig. 51, 635–668 (2021). https://doi.org/10.1007/s40005-021-00529-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-021-00529-9