Abstract
Background:
Inflammatory bowel disease (IBD) is an incurable disease that negatively influences the quality of life of patients. Current and emerging therapies target proinflammatory cytokines and/or receptors to downregulate proinflammatory responses, but insufficient remission requires other therapeutic agents. Herein, we report that the synthetic anti-inflammatory peptide 15 (SAP15) is capable of cell penetration and anti-inflammatory activity in human macrophages.
Methods:
SAP15 was labeled with fluorescence and administered to human leukemia monocytic cells (THP-1) cells for cell penetration analysis. Using biolayer interferometry analysis, the binding affinity of SAP15 with histone deacetylase 5 (HDAC5) was measured. SAP15-treated THP-1 cells were analyzed by protein phosphorylation assay, flow cytometry, and enzyme-linked immunosorbent assay (ELISA). In addition, in vivo analysis of the therapeutic effect on IBD was observed in a dextran sulfate sodium (DSS)-induced model. Samples from SAP15-treated mice were analyzed at both the macroscopic and microscopic levels using ELISA, myeloperoxidase (MPO) assays, and histological evaluations.
Results:
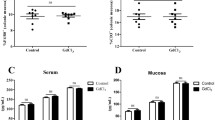
SAP15 was internalized within the cytosol and nucleus of THP-1 cells and bound to the HDAC5 protein. SAP15-treated macrophages were assessed for protein phosphorylation and showed inhibited phosphorylation of HDAC5 and other immune-related proteins, which led to increased M2-like macrophage markers and decreased M1-like macrophage markers and tumor necrosis factor-α and interleukin-6 cytokine levels. The SAP15 treatment on IBD model showed significant recovery of colon length. Further histological analysis of colon demonstrated the therapeutic effect of SAP15 on mucosal layer. Moreover, proinflammatory cytokine levels and MPO activity from the plasma show that SAP15 is effective in reduced proinflammatory responses.
Conclusion:
These findings suggest that SAP15 is a novel peptide with a novel cell-penetrating peptide with anti-inflammatory property that can be used as a therapeutic agent for IBD and other inflammatory diseases.







Similar content being viewed by others
References
Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–21.e2.
Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–78.
Barreiro-de Acosta M, Alvarez Castro A, Souto R, Iglesias M, Lorenzo A, Dominguez-Muñoz JE. Emigration to western industrialized countries: a risk factor for developing inflammatory bowel disease. J Crohns Colitis. 2011;5:566–9.
Cui G, Yuan A. A systematic review of epidemiology and risk factors associated with chinese inflammatory bowel disease. Front Med (Lausanne). 2018;5:183.
Muegge BD, Kuczynski J, Knights D, Clemente JC, González A, Fontana L, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–4.
de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13:13–27.
Klein A, Eliakim R. Non steroidal anti-inflammatory drugs and inflammatory bowel disease. Pharmaceuticals (Basel). 2010;3:1084–92.
Takeuchi K, Smale S, Premchand P, Maiden L, Sherwood R, Thjodleifsson B, et al. Prevalence and mechanism of nonsteroidal anti-inflammatory drug-induced clinical relapse in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:196–202.
Gisbert JP, Gomollón F, Maté J, Pajares JM. Role of 5-aminosalicylic acid (5-ASA) in treatment of inflammatory bowel disease: a systematic review. Dig Dis Sci. 2002;47:471–88.
Kim MJ, Choe YH. Monitoring and safety of azathioprine therapy in inflammatory bowel disease. Pediatr Gastroenterol Hepatol Nutr. 2013;16:65–70.
Weissman S, Chris-Olaiya A, Mehta TI, Aziz M, Alshati A, Berry R, et al. A novel player: cyclosporine therapy in the management of inflammatory bowel disease. Transl Gastroenterol Hepatol. 2019;4:67.
Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004;2:731–43.
Sandborn WJ. The present and future of inflammatory bowel disease treatment. Gastroenterol Hepatol (N Y). 2016;12:438–41.
Triantafillidis JK, Merikas E, Georgopoulos F. Current and emerging drugs for the treatment of inflammatory bowel disease. Drug Des Devel Ther. 2011;5:185–210.
Papamichael K, Lin S, Moore M, Papaioannou G, Sattler L, Cheifetz AS. Infliximab in inflammatory bowel disease. Ther Adv Chronic Dis. 2019;10:2040622319838443.
Wasan SK, Kane SV. Adalimumab for the treatment of inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2011;5:679–84.
Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:269–78.
Weisshof R, El Jurdi K, Zmeter N, Rubin DT. Emerging therapies for inflammatory bowel disease. Adv Ther. 2018;35:1746–62.
Hazel K, O’Connor A. Emerging treatments for inflammatory bowel disease. Ther Adv Chronic Dis. 2020;11:2040622319899297.
Flanagan ME, Blumenkopf TA, Brissette WH, Brown MF, Casavant JM, Shang-Poa C, et al. Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem. 2010;53:8468–84.
Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–24.
Danese S, Argollo M, Le Berre C, Peyrin-Biroulet L. JAK selectivity for inflammatory bowel disease treatment: does it clinically matter? Gut. 2019;68:1893–9.
Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16:843–62.
Sandborn WJ, Ghosh S, Panes J, Vranic I, Wang W, Niezychowski W. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:1485–93.e2.
Panés J, Sandborn WJ, Schreiber S, Sands BE, Vermeire S, D’Haens G, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66:1049–59.
Mohammadi A, Sharifi A, Pourpaknia R, Mohammadian S, Sahebkar A. Manipulating macrophage polarization and function using classical HDAC inhibitors: implications for autoimmunity and inflammation. Crit Rev Oncol Hematol. 2018;128:1–18.
Huang HM, Fan SJ, Zhou XR, Liu YJ, Li X, Liao LP, et al. Histone deacetylase inhibitor givinostat attenuates nonalcoholic steatohepatitis and liver fibrosis. Acta Pharmacol Sin. 2022;43:941–53.
Henninot A, Collins JC, Nuss JM. The current state of peptide drug discovery: back to the future? J Med Chem. 2018;61:1382–414.
Muttenthaler M, King GF, Adams DJ, Alewood PF. Trends in peptide drug discovery. Nat Rev Drug Discov. 2021;20:309–25.
Lee AC, Harris JL, Khanna KK, Hong JH. A comprehensive review on current advances in peptide drug development and design. Int J Mol Sci. 2019;20:309–25.
Sun X, Guo C, Zhao F, Zhu J, Xu Y, Liu ZQ, et al. Vasoactive intestinal peptide stabilizes intestinal immune homeostasis through maintaining interleukin-10 expression in regulatory B cells. Theranostics. 2019;9:2800–11.
Salaga M, Binienda A, Draczkowski P, Kosson P, Kordek R, Jozwiak K, et al. Novel peptide inhibitor of dipeptidyl peptidase IV (Tyr-Pro-D-Ala-NH2 with anti-inflammatory activity in the mouse models of colitis. Peptides. 2018;108:34–45.
Choi DH, Lee D, Jo BS, Park KS, Lee KE, Choi JK, et al. A Synthetic cell-penetrating heparin-binding peptide derived from BMP4 with anti-inflammatory and chondrogenic functions for the treatment of arthritis. Int J Mol Sci. 2020;21:4251.
Jo BS, Lee Y, Suh JS, Park YS, Lee HJ, Lee JY, et al. A novel calcium-accumulating peptide/gelatin in situ forming hydrogel for enhanced bone regeneration. J Biomed Mater Res A. 2018;106:531–42.
Lee D, Park KS, Yoon GJ, Lee HJ, Lee JY, Park YS, et al. Identification of cell-penetrating osteogenic peptide from copine-7 protein and its delivery system for enhanced bone formation. J Biomed Mater Res A. 2019;107:2392–402.
Lee JY, Suh JS, Kim JM, Kim JH, Park HJ, Park YJ, et al. Identification of a cell-penetrating peptide domain from human beta-defensin 3 and characterization of its anti-inflammatory activity. Int J Nanomedicine. 2015;10:5423–34.
Lee JY, Lee DW, Jo BS, Park KS, Park YS, Chung CP, et al. Engineered synthetic cell penetrating peptide with intracellular anti-inflammatory bioactivity: an in vitro and in vivo study. J Biomed Mater Res A. 2021;109:2001–16.
Lee JY, Seo YN, Park HJ, Park YJ, Chung CP. The cell-penetrating peptide domain from human heparin-binding epidermal growth factor-like growth factor (HB-EGF) has anti-inflammatory activity in vitro and in vivo. Biochem Biophys Res Commun. 2012;419:597–604.
Amblard M, Fehrentz JA, Martinez J, Subra G. Methods and protocols of modern solid phase peptide synthesis. Mol Biotechnol. 2006;33:239–54.
Starr T, Bauler TJ, Malik-Kale P, Steele-Mortimer O. The phorbol 12-myristate-13-acetate differentiation protocol is critical to the interaction of THP-1 macrophages with Salmonella Typhimurium. PLoS One. 2018;13: e0193601.
Surdziel E, Clay I, Nigsch F, Thiemeyer A, Allard C, Hoffman G, et al. Multidimensional pooled shRNA screens in human THP-1 cells identify candidate modulators of macrophage polarization. PLoS One. 2017;12: e0183679.
Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci. 2014;10:520–9.
Dhople V, Krukemeyer A, Ramamoorthy A. The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim Biophys Acta. 2006;1758:1499–512.
Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005;6:551–7.
Hirsch T, Spielmann M, Zuhaili B, Fossum M, Metzig M, Koehler T, et al. Human beta-defensin-3 promotes wound healing in infected diabetic wounds. J Gene Med. 2009;11:220–8.
Patel SG, Sayers EJ, He L, Narayan R, Williams TL, Mills EM, et al. Cell-penetrating peptide sequence and modification dependent uptake and subcellular distribution of green florescent protein in different cell lines. Sci Rep. 2019;9:6298.
van den Berg A, Dowdy SF. Protein transduction domain delivery of therapeutic macromolecules. Curr Opin Biotechnol. 2011;22:888–93.
Lim S, Lee JA, Koo JH, Kang TG, Ha SJ, Choi JM. Cell type preference of a novel human derived cell-permeable peptide dNP2 and TAT in murine splenic immune cells. PLoS One. 2016;11: e0155689.
Drescher DG, Ramakrishnan NA, Drescher MJ. Surface plasmon resonance (SPR) analysis of binding interactions of proteins in inner-ear sensory epithelia. Methods Mol Biol. 2009;493:323–43.
Leonard P, Säfsten P, Hearty S, McDonnell B, Finlay W, O’Kennedy R. High throughput ranking of recombinant avian scFv antibody fragments from crude lysates using the Biacore A100. J Immunol Methods. 2007;323:172–9.
Murali S, Rustandi RR, Zheng X, Payne A, Shang L. Applications of surface plasmon resonance and biolayer interferometry for virus-ligand binding. Viruses. 2022;14:717.
Abdiche YN, Malashock DS, Pons J. Probing the binding mechanism and affinity of tanezumab, a recombinant humanized anti-NGF monoclonal antibody, using a repertoire of biosensors. Protein Sci. 2008;17:1326–35.
Shibata H, Nishimura K, Miyama C, Tada M, Suzuki T, Saito Y, et al. Comparison of different immunoassay methods to detect human anti-drug antibody using the WHO erythropoietin antibody reference panel for analytes. J Immunol Methods. 2018;452:73–7.
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64.
Genin M, Clement F, Fattaccioli A, Raes M, Michiels C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer. 2015;15:577.
Yao Y, Xu XH, Jin L. Macrophage polarization in physiological and pathological pregnancy. Front Immunol. 2019;10:792.
Porta C, Riboldi E, Ippolito A, Sica A. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol. 2015;27:237–48.
Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Front Immunol. 2014;5:614.
Chami B, Martin NJJ, Dennis JM, Witting PK. Myeloperoxidase in the inflamed colon: a novel target for treating inflammatory bowel disease. Arch Biochem Biophys. 2018;645:61–71.
Breckwoldt MO, Chen JW, Stangenberg L, Aikawa E, Rodriguez E, Qiu S, et al. Tracking the inflammatory response in stroke in vivo by sensing the enzyme myeloperoxidase. Proc Natl Acad Sci U S A. 2008;105:18584–9.
Wehkamp J, Fellermann K, Herrlinger KR, Bevins CL, Stange EF. Mechanisms of disease: defensins in gastrointestinal diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:406–15.
Coretti L, Natale A, Cuomo M, Florio E, Keller S, Lembo F, et al. The interplay between defensins and microbiota in crohn’s disease. Mediators Inflamm. 2017;2017:8392523.
Acknowledgement
This study was supported in part by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1A6A1A03039462).
Author information
Authors and Affiliations
Contributions
Conceptualization and draft supervision of project: JYL, YSP, CPC, and YJP. Overall experiments and preparation of the original draft: DK Writing the manuscript: DK, JYL, and YJP. Biological experiments: DWL, and GY. Peptide synthesis: EKJ, MSC, HCL. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Seoul National University (IACUC Approval No. SNU-190121-1).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, D., Lee, D.W., Yoon, G. et al. Therapeutic Effect of HDAC5 Binding and Cell Penetrating Peptide for the Treatment of Inflammatory Bowel Disease. Tissue Eng Regen Med 20, 965–979 (2023). https://doi.org/10.1007/s13770-023-00572-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-023-00572-7