Abstract
Catalytic nanoparticles with natural enzyme-mimicking properties, known as nanozymes, have emerged as excellent candidate materials for cancer immunotherapy. Owing to their enzymatic activities, artificial nanozymes not only serve as responsive carriers to load drugs and therapeutic molecules for cancer treatment, but also act as enzymes for modulating the immunosuppression of the tumor microenvironment (TME) via the catalytic activities of catalase, peroxidase, superoxide dismutase, and oxidase. The immunosuppressive pro-tumor TME can be reversed to the immunoactive anti-tumor TME by utilizing both reactive oxygen species (ROS)-generating and ROS-scavenging nanozymes, which enhance the efficacy of cancer immunotherapy. In this review, we introduce representative ROS-generating and ROS-scavenging nanozymes and discuss how artificial nanozymes respond to the conditions of the TME. Based on the mutual interaction between nanozymes and TME, recent therapeutic pathways to provoke anti-cancer immune responses using nanozymes are discussed.





Reproduced from [51] with permission from Wiley–VCH. Copyright 2021

Reproduced from [52] with permission from Wiley–VCH. Copyright 2020

Reproduced from [53] with permission from Elsevier. Copyright 2018

Reproduced from [55] with permission from the American Chemical Society (ACS). Copyright 2020

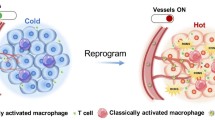
Reproduced from [61] with permission from Nature. Copyright 2017

Reproduced from [63] with permission from Springer. Copyright 2019

Reproduced from [62] with permission from Wiley–VCH. Copyright 2020
Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Zhu G, Lynn GM, Jacobson O, Chen K, Liu Y, Zhang H, et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat Commun. 2017;8:1954.
Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16:489–96.
Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat Biotechnol. 2015;33:64–72.
Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–60.
Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–81.
Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501.
Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A. 2014;111:11774–9.
Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–61.
Zhao J, Wen X, Tian L, Li T, Xu C, Wen X, et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat Commun. 2019;10:899.
Chen Q, Hu Q, Dukhovlinova E, Chen G, Ahn S, Wang C, et al. Photothermal therapy promotes tumor infiltration and antitumor activity of CAR T cells. Adv Mater. 2019;31:e1900192.
Mohammed S, Sukumaran S, Bajgain P, Watanabe N, Heslop HE, Rooney CM, et al. Improving chimeric antigen receptor-modified T cell function by reversing the immunosuppressive tumor microenvironment of pancreatic cancer. Mol Ther. 2017;25:249–58.
Maus MV, June CH. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin Cancer Res. 2016;22:1875–84.
Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of Glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375:2561–9.
Nguyen TL, Yin Y, Choi Y, Jeong JH, Kim J. Enhanced cancer DNA vaccine via direct transfection to host dendritic cells recruited in injectable scaffolds. ACS Nano. 2020;14:11623–36.
Cha BG, Jeong JH, Kim J. Extra-large pore mesoporous silica nanoparticles enabling co-delivery of high amounts of protein antigen and toll-like receptor 9 agonist for enhanced cancer vaccine efficacy. ACS Cent Sci. 2018;4:484–92.
Nguyen TL, Cha BG, Choi Y, Im J, Kim J. Injectable dual-scale mesoporous silica cancer vaccine enabling efficient delivery of antigen/adjuvant-loaded nanoparticles to dendritic cells recruited in local macroporous scaffold. Biomaterials. 2020;239:119859.
Lee JY, Kim MK, Nguyen TL, Kim J. Hollow mesoporous silica nanoparticles with extra-large mesopores for enhanced cancer vaccine. ACS Appl Mater Interfaces. 2020;12:34658–66.
Kerr MD, McBride DA, Chumber AK, Shah NJ. Combining therapeutic vaccines with chemo- and immunotherapies in the treatment of cancer. Expert Opin Drug Discov. 2021;16:89–99.
Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Chapter seven - therapeutic cancer vaccines: past, present, and future. In: Tew KD, Fisher PB, editors. Advances in Cancer Research. Cambridge: Academic Press; 2013. p. 421–75.
Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer? BMC Med. 2016;14:73.
Duan X, Chan C, Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed Engl. 2019;58:670–80.
Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–51.
Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–77.
Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168–82.
Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–24.
Ma J, Qiu J, Wang S. Nanozymes for catalytic cancer immunotherapy. ACS Appl Nano Mater. 2020;3:4925–43.
Ying JF, Lu ZB, Fu LQ, Tong Y, Wang Z, Li WF, et al. The role of iron homeostasis and iron-mediated ROS in cancer. Am J Cancer Res. 2021;11:1895–912.
Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Can Res. 2014;74:104–18
Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci. 2014;1319:47–65.
Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419–30.
Meng X, Li D, Chen L, He H, Wang Q, Hong C, et al. High-performance self-cascade pyrite nanozymes for apoptosis-ferroptosis synergistic tumor therapy. ACS Nano. 2021;15:5735–51.
Wang Q, Niu D, Shi J, Wang L. A three-in-one ZIFs-derived CuCo(O)/GOx@PCNs hybrid cascade nanozyme for immunotherapy/enhanced starvation/photothermal therapy. ACS Appl Mater Interfaces. 2021;13:11683–95.
Zhou K, Cheng T, Zhan J, Peng X, Zhang Y, Wen J, et al. Targeting tumor-associated macrophages in the tumor microenvironment. Oncol Lett. 2020;20:234.
Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11:583084.
Umansky V, Blattner C, Fleming V, Hu X, Gebhardt C, Altevogt P, et al. Myeloid-derived suppressor cells and tumor escape from immune surveillance. Semin Immunopathol. 2017;39:295–305.
Wei T, Zhong W, Li Q. Role of heterogeneous regulatory T cells in the tumor microenvironment. Pharmacol Res. 2020;153:104659.
Chaudhary B, Elkord E. Regulatory T cells in the tumor microenvironment and cancer progression: role and therapeutic targeting. Vaccines (Basel). 2016;4:28.
Liu C, Workman CJ, Vignali DA. Targeting regulatory T cells in tumors. FEBS J. 2016;283:2731–48.
Beyer M, Schultze LJ. Regulatory T cells: major players in the tumor microenvironment. Curr Pharm Des. 2009;15:1879–92.
Zhang L, Wang S, Wang Y, Zhao W, Zhang Y, Zhang N, et al. Effects of hypoxia in intestinal tumors on immune cell behavior in the tumor microenvironment. Front Immunol. 2021;12:645320.
Li Y, Zhao L, Li XF. Hypoxia and the Tumor microenvironment. Technol Cancer Res Treat. 2021;20:15330338211036304.
Vito A, El-Sayes N, Mossman K. Hypoxia-driven immune escape in the tumor microenvironment. Cells. 2020;9:992.
Bohme I, Bosserhoff AK. Acidic tumor microenvironment in human melanoma. Pigment Cell Melanoma Res. 2016;29:508–23.
Sitkovsky MV, Hatfield S, Abbott R, Belikoff B, Lukashev D, Ohta A. Hostile, hypoxia–A2-adenosinergic tumor biology as the next Barrier to overcome for tumor immunologists. Cancer Immunol Res. 2014;2:598–605.
Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014;74:665–74.
Aboelella NS, Brandle C, Kim T, Ding ZC, Zhou G. Oxidative stress in the tumor microenvironment and its relevance to cancer immunotherapy. Cancers. 2021;13:986.
Wang H, Cheng L, Ma S, Ding L, Zhang W, Xu Z, et al. Self-assembled multiple-enzyme composites for enhanced synergistic cancer starving-catalytic therapy. ACS Appl Mater Interfaces. 2020;12:20191–201.
Chen M, Deng G, He Y, Li X, Liu W, Wang W, et al. Ultrasound-enhanced generation of reactive oxygen species for MRI-guided tumor therapy by the Fe@Fe3O4-based peroxidase-mimicking nanozyme. ACS Appl Bio Mater. 2019;3:639–47.
Lan M, Zhao S, Liu W, Lee CS, Zhang W, Wang P. Photosensitizers for photodynamic therapy. Adv Healthc Mater. 2019;8:e1900132.
Zhu L, Liu J, Zhou G, Liu TM, Dai Y, Nie G, et al. Remodeling of tumor microenvironment by tumor-targeting Nanozymes enhances immune activation of CAR T cells for combination therapy. Small. 2021;17:e2102624.
Zhao Y, Xiao X, Zou M, Ding B, Xiao H, Wang M, et al. Nanozyme-initiated in situ cascade reactions for self-amplified biocatalytic immunotherapy. Adv Mater. 2021;33:e2006363.
Liang R, Liu L, He H, Chen Z, Han Z, Luo Z, et al. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials. 2018;177:149–60.
Gao L, Liu R, Gao F, Wang Y, Jiang X, Gao X. Plasmon-mediated generation of reactive oxygen species from near-infrared light excited gold nanocages for photodynamic therapy in vitro. ACS Nano. 2014;8:7260–71.
Liu Y, Zhen W, Wang Y, Song S, Zhang H. Na2S2O8 nanoparticles trigger antitumor immunotherapy through reactive oxygen species storm and surge of tumor osmolarity. J Am Chem Soc. 2020;142:21751–7.
Tang F, Du X, Liu M, Zheng P, Liu Y. Anti-CTLA-4 antibodies in cancer immunotherapy: selective depletion of intratumoral regulatory T cells or checkpoint blockade? Cell Biosci. 2018;8:30.
Chen H, Luan X, Paholak HJ, Burnett JP, Stevers NO, Sansanaphongpricha K, et al. Depleting tumor-associated Tregs via nanoparticle-mediated hyperthermia to enhance anti-CTLA-4 immunotherapy. Nanomedicine (Lond). 2019;15:77–92.
Yao J, Cheng Y, Zhou M, Zhao S, Lin S, Wang X, et al. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem Sci. 2018;9:2927–33.
Kaizer J, Csay T, Kővári P, Speier G, Párkányi L. Catalase mimics of a manganese(II) complex: the effect of axial ligands and pH. J Mol Catal A Chem. 2008;280:203–9.
Singh N, Savanur MA, Srivastava S, D’Silva P, Mugesh G. A manganese oxide nanozyme prevents the oxidative damage of biomolecules without affecting the endogenous antioxidant system. Nanoscale. 2019;11:3855–63.
Yang G, Xu L, Chao Y, Xu J, Sun X, Wu Y, et al. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun. 2017;8:902.
Xu B, Cui Y, Wang W, Li S, Lyu C, Wang S, et al. Immunomodulation-enhanced nanozyme-based tumor catalytic therapy. Adv Mater. 2020;32:e2003563.
Wang J, Fang L, Li P, Ma L, Na W, Cheng C, et al. Inorganic nanozyme with combined self-oxygenation/degradable capabilities for sensitized cancer immunochemotherapy. Nanomicro Lett. 2019;11:74.
Acknowledgements
This research was supported by grant from the National Research Foundation (NRF) of Korea (No. 2020M3A9D3039720) funded by the Korean government (MSIT) and the R&D Program for Forest Science Technology (No. 2020209B10-2222-BA01) provided by the Korea Forest Service (KFS) of the Korea Forestry Promotion Institute. Ngoc Man Phan and Thanh Loc Nguyen contributed equally to this work. All authors approved the final version of the manuscript. Correspondence should be addressed to Jaeyun Kim.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no competing financial interest.
Ethical Statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Phan, N.M., Nguyen, T.L. & Kim, J. Nanozyme-Based Enhanced Cancer Immunotherapy. Tissue Eng Regen Med 19, 237–252 (2022). https://doi.org/10.1007/s13770-022-00430-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00430-y