Abstract
Background:
The extracellular matrix (ECM) of articular cartilage has an inhibitory effect on vascularization, yet clinical utilization has been technically challenging. In this study, we aimed to fabricate a biologically functional ECM powder suspension from porcine articular cartilage that inhibits neovascularization (NV).
Methods:
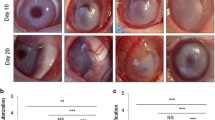
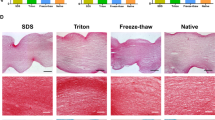
The digested-cartilage acellular matrix (dg-CAM) was prepared by sequential processes of decellularization, enzymatic digestion and pulverization. Physicochemical properties of dg-CAM were compared with that of native cartilage tissue (NCT). Cellular interactions between human umbilical vein endothelial cells (HUVECs) and dg-CAM was evaluated with proliferation, migration and tube formation assays compared with that of type I collagen (COL) and bevacizumab, an anti-angiogenic drug. We then investigated the therapeutic potential of topical administration of dg-CAM suspension on the experimentally induced rabbit corneal NV model.
Results:
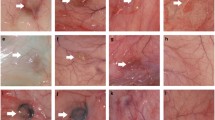
The dg-CAM released a significantly larger amount of soluble proteins than that of the NCT and showed an improved hydrophilic and dispersion properties. In contrast, the dg-CAM contained a large amount of collagen, glycosaminoglycans and anti-angiogenic molecules as much as the NCT. The inhibitory effect on NV of the dg-CAM was more prominent than that of COL and even comparable to that of bevacizumab in inhibiting the HUVECs. The therapeutic potential of the dg-CAM was comparable to that of bevacizumab in the rabbit corneal NV model by efficiently inhibiting neovessel formation of the injured cornea.
Conclusion:
The current study developed a dg-CAM having anti-angiogenic properties, together with water-dispersible properties suitable for topical or minimally invasive application for prevention of vessel invasion.








Similar content being viewed by others
References
Taylor DA, Sampaio LC, Ferdous Z, Gobin AS, Taite LJ. Decellularized matrices in regenerative medicine. Acta Biomater. 2018;74:74–89.
Luo JC, Chen W, Chen XH, Qin TW, Huang YC, Xie HQ, et al. A multi-step method for preparation of porcine small intestinal submucosa (SIS). Biomaterials. 2011;32:706–13.
Agrawal V, Tottey S, Johnson SA, Freund JM, Siu BF, Badylak SF. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng Part A. 2011;17:2435–43.
Huleihel L, Bartolacci JG, Dziki JL, Vorobyov T, Arnold B, Scarritt ME, et al. Matrix-bound nanovesicles recapitulate extracellular matrix effects on macrophage phenotype. Tissue Eng Part A. 2017;23:1283–94.
Patra D, Sandell LJ. Antiangiogenic and anticancer molecules in cartilage. Expert Rev Mol Med. 2012;14:e10.
Eisenstein R, Sorgente N, Soble LW, Miller A, Kuettner KE. The resistance of certain tissues to invasion: penetration of explanted tissues by vascularized mesenchyme. Am J Pathol. 1973;73:765–74.
Brem H, Folkmant J. Inhibition of tumor angiogenesis mediated by cartilage. J Exp Med. 1975;141:427–39.
Chen CC, Liao CH, Wang YH, Hsu YM, Huang SH, Chang CH, et al. Cartilage fragments from osteoarthritic knee promote chondrogenesis of mesenchymal stem cells without exogenous growth factor induction. J Orthop Res. 2012;30:393–400.
Yang Q, Peng J, Guo Q, Huang J, Zhang L, Yao J, et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials. 2008;29:2378–87.
Utomo L, Pleumeekers MM, Nimeskern L, Nürnberger S, Stok KS, Hildner F, et al. Preparation and characterization of a decellularized cartilage scaffold for ear cartilage reconstruction. Biomed Mater. 2015;10:015010.
Hiraki Y, Inoue H, Iyama K, Kamizono A, Ochiai M, Shukunami C, et al. Identification of chondromodulin I as a novel endothelial cell growth inhibitor. J Biol Chem. 1997;272:32419–26.
Wang Z, Bryan J, Franz C, Havlioglu N, Sandell LJ. Type IIB procollagen NH2-propeptide induces death of tumor cells via interaction with integrins αvβ3 and αvβ5. J Biol Chem. 2010;285:20806–17.
Kobayashi T, Kakizaki I, Nozaka H, Nakamura T. Chondroitin sulfate proteoglycans from salmon nasal cartilage inhibit angiogenesis. Biochem Biophys Rep. 2017;9:72–8.
Chlenski A, Liu S, Crawford SE, Volpert OV, DeVries GH, Evangelista A, et al. SPARC is a key schwannian-derived inhibitor controlling neuroblastoma tumor angiogenesis. Cancer Res. 2002;62:7357–63.
González RP, Leyva A, Moraes MO. Shark cartilage as source of antiangiogenic compounds: from basic to clinical research. Biol Pharm Bull. 2001;24:1097–101.
Jiao B, Chen J, Miao W, Wang L, Zhu Y, Miao H. Identification and biological characterization of angiogenic and tumor growth inhibitors derived from sinica cetorhinus maximum cartilage. Mar Drugs. 2004;2:30–8.
Zheng L, Ling P, Wang Z, Niu R, Hu C, Zhang T, et al. A novel polypeptide from shark cartilage with potent anti-angiogenic activity. Cancer Biol Ther. 2007;6:775–80.
Sanz L, Álvarez-Vallina L. The extracellular matrix: a new turn-of-the-screw for anti-angiogenic strategies. Trends Mol Med. 2003;9:256–62.
Spang MT, Christman KL. Extracellular matrix hydrogel therapies: in vivo applications and development. Acta Biomater. 2018;68:1–14.
Manni ML, Czajka CA, Oury TD, Gilbert TW. Extracellular matrix powder protects against bleomycin-induced pulmonary fibrosis. Tissue Eng Part A. 2011;17:2795–804.
Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605–14.
Sicari BM, Dziki JL, Siu BF, Medberry CJ, Dearth CL, Badylak SF. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials. 2014;35:8605–12.
Jung CS, Kim BK, Lee J, Min BH, Park SH. Development of printable natural cartilage matrix bioink for 3D printing of irregular tissue shape. Tissue Eng Regen Med. 2018;15:155–62.
Mudalal S, Babini E, Cavani C, Petracci M. Quantity and functionality of protein fractions in chicken breast fillets affected by white striping. Poult Sci. 2014;93:2108–16.
Choi KH, Song BR, Choi BH, Lee M, Park SR, Min BH. Cartilage tissue engineering using chondrocyte-derived extracellular matrix scaffold suppressed vessel invasion during chondrogenesis of mesenchymal stem cells in vivo. Tissue Eng Regen Med. 2012;9:43–50.
DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, et al. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One. 2010;5:e13039.
Kim WJ, Jeong HO, Chung SK. The effect of bevacizumab on corneal neovascularization in rabbits. Korean J Ophthalmol. 2010;24:230–6.
Ozdemir O, Altintas O, Altintas L, Ozkan B, Akdag C, Yüksel N. Comparison of the effects of subconjunctival and topical anti-VEGF therapy (bevacizumab) on experimental corneal neovascularization. Arq Bras Oftalmol. 2014;77:209–13.
Dastjerdi MH, Al-Arfaj KM, Nallasamy N, Hamrah P, Jurkunas UV, Pineda R 2nd, et al. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, non-comparative study. Arch Ophthalmol. 2009;127:381–9.
Sotozono C, Ang LP, Koizumi N, Higashihara H, Ueta M, Inatomi T, et al. New grading system for the evaluation of chronic ocular manifestations in patients with Stevens–Johnson syndrome. Ophthalmology. 2007;114:1294–302.
Yadav T, Mungray AA, Mungray AK. A comparative analysis of a TiO2 nanoparticle dispersion in various biological extracts. RSC Adv. 2015;5:64421–32.
Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43.
Tantra R, Jing S, Pichaimuthu SK, Walker N, Noble J, Hackley VA. Dispersion stability of nanoparticles in ecotoxicological investigations: the need for adequate measurement tools. J Nanopart Res. 2011;13:3765–80.
Zako T, Nagata H, Terada N, Sakono M, Soga K, Maeda M. Improvement of dispersion stability and characterization of upconversion nanophosphors covalently modified with PEG as a fluorescence bioimaging probe. J Mater Sci. 2008;43:5325–30.
Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems—a review (Part 2). Trop J Pharm Res. 2013;12:265–73.
Hong T, Iwashita K, Shiraki K. Viscosity control of protein solution by small solutes: a review. Curr Protein Pept Sci. 2018;19:746–58.
Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34.
Zhong Y, Jiang A, Sun F, Xiao Y, Gu Y, Wu L, et al. A comparative study of the effects of different decellularization methods and genipin-cross-linking on the properties of tracheal matrices. Tissue Eng Regen Med. 2019;16:39–50.
Singh S, Afara IO, Tehrani AH, Oloyede A. Effect of decellularization on the load-bearing characteristics of articular cartilage matrix. Tissue Eng Regen Med. 2015;12:294–305.
Pati F, Jang J, Ha DH, Kim SW, Rhie JW, Shim JH, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935.
Rothrauff BB, Coluccino L, Gottardi R, Ceseracciu L, Scaglione S, Goldoni L, et al. Efficacy of thermoresponsive, photocrosslinkable hydrogels derived from decellularized tendon and cartilage extracellular matrix for cartilage tissue engineering. J Tissue Eng Regen Med. 2018;12:e159–70.
Neve A, Cantatore FP, Maruotti N, Corrado A, Ribatti D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed Res Int. 2014;2014:756078.
Boivin D, Gendron S, Beaulieu E, Gingras D, Béliveau R. The antiangiogenic agent neovastat (AE-941) induces endothelial cell apoptosis. Mol Cancer Ther. 2002;1:795–802.
Choi BH, Choi KH, Lee HS, Song BR, Park SR, Yang JW, et al. Inhibition of blood vessel formation by a chondrocyte-derived extracellular matrix. Biomaterials. 2014;35:5711–20.
Cunniffe GM, Díaz-payno PJ, Sheehy EJ, Critchley SE, Almeida HV, Pitacco P, et al. Tissue-specific extracellular matrix scaffolds for the regeneration of spatially complex musculoskeletal tissues. Biomaterials. 2019;188:63–73.
French KM, Boopathy AV, DeQuach JA, Chingozha L, Lu H, Christman KL, et al. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater. 2012;8:4357–64.
Crapo PM, Medberry CJ, Reing JE, Tottey S, van der Merwe Y, Jones KE, et al. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials. 2012;33:3539–47.
Gingras D, Boivin D, Deckers C, Gendron S, Barthomeuf C, Béliveau R. Neovastat-a novel antiangiogenic drug for cancer therapy. Anticancer Drugs. 2003;14:91–6.
Lee HS, Lee JH, Kim CE, Yang JW. Anti-neovascular effect of chondrocyte-derived extracellular matrix on corneal alkaline burns in rabbits. Graefes Arch Clin Exp Ophthalmol. 2014;252:951–61.
Belting M. Glycosaminoglycans in cancer treatment. Thromb Res. 2014;133:S95–101.
Delaney CE, Weagant BT, Addison CL. The inhibitory effects of endostatin on endothelial cells are modulated by extracellular matrix. Exp Cell Res. 2006;312:2476–89.
Abdollahi A, Hlatky L, Huber PE. Endostatin: the logic of antiangiogenic therapy. Drug Resist Updat. 2005;8:59–74.
Jiménez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–8.
Hasselaar P, Sage EH. SPARC antagonizes the effect of basic fibroblast growth factor on the migration of bovine aortic endothelial cells. J Cell Biochem. 1992;49:272–83.
Raines EW, Lanes TF, Iruela-arispet ML, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF) -AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci U S A. 1992;89:1281–5.
Abdelfattah NS, Amgad M, Zayed AA, Hussein H, EI-Baky NA. Molecular underpinnings of corneal angiogenesis: advances over the past decade. Int J Ophthalmol. 2016;9:768–79.
Kim SW, Ha BJ, Kim EK, Tchah H, Kim T. The Effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008;115:e33–8.
Acknowledgement
This study was supported by a grant from the Korea Health Technology R&D Project (HI14C0744 and HI17C2191) through the Korea Health Industry Development Institute funded by the Ministry of Health & Welfare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
The animal study was approved by the Laboratory Animal Research Center of Ajou University Medical Center (No, 2013-0014) and performed according to the regulations of the ARVO statement for Ophthalmic and Visual research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yun, HW., Choi, B.H., Park, D.Y. et al. Inhibitory Effect of Topical Cartilage Acellular Matrix Suspension Treatment on Neovascularization in a Rabbit Corneal Model. Tissue Eng Regen Med 17, 625–640 (2020). https://doi.org/10.1007/s13770-020-00275-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00275-3