Abstract
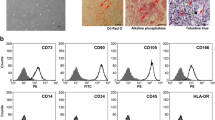
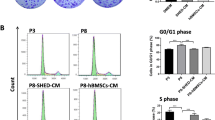
Atmospheric (in vitro) oxygen pressure is around 150 mm Hg (20% O2), whereas physiologic (in vivo) oxygen pressure ranges between 5 and 50 mm Hg (0.7–7% O2). The normoxic environment in cell culture does not refer to a physiological stem cell niche. The aim of this study is to investigate the effect of oxygen concentration on cell properties of human mesenchymal stem cells (MSCs). We analyzed cell proliferation rate, senescence, immunophenotype, stemness gene expression and differentiation potency with human urine stem cells (USCs), dental pulp stem cells (DPSCs), amniotic fluid stem cells (AFSCs), and bone marrow stromal cells (BMSCs). USCs, DPSCs, AFSCs and BMSCs were cultured under either 5% O2 hypoxic or 20% O2 normoxic conditions for 5 days. MSCs cultured under hypoxia showed significantly increased proliferation rate and high percentage of S-phase cells, compared to normoxic condition. In real-time PCR assay, the cells cultured under hypoxia expressed higher level of Oct4, C-Myc, Nanog, Nestin and HIF-1α. In immunophenotype analysis, MSCs cultured under hypoxia maintained higher level of the MSC surface markers, and lower hematopoietic markers. Senescence was inhibited under hypoxia. Hypoxia enhances osteogenic differentiation efficiency compared to normoxia. Hypoxia showed enhanced cell proliferation rate, retention of stem cell properties, inhibition of senescence, and increased differentiation ability compared to normoxia.





Similar content being viewed by others
References
Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88:792–806.
Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20.
Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood). 2001;226:507–20.
Kaviani A, Guleserian K, Perry TE, Jennings RW, Ziegler MM, Fauza DO. Fetal tissue engineering from amniotic fluid. J Am Coll Surg. 2003;196:592–7.
Chun SY, Kim HT, Lee JS, Kim MJ, Kim BS, Kim BW, et al. Characterization of urine-derived cells from upper urinary tract in patients with bladder cancer. Urology. 2012;79(1186):e1181–7.
Thakker R, Yang P. Mesenchymal stem cell therapy for cardiac repair. Curr Treat Options Cardiovasc Med. 2014;16:323.
Dadon-Nachum M, Melamed E, Offen D. Stem cells treatment for sciatic nerve injury. Expert Opin Biol Ther. 2011;11:1591–7.
Fan L, Liu R, Li J, Shi Z, Dang X, Wang K. Low oxygen tension enhances osteogenic potential of bone marrow-derived mesenchymal stem cells with osteonecrosis-related functional impairment. Stem Cells Int. 2015;2015:950312.
Khan M, Akhtar S, Mohsin S, Khan N, Riazuddin S. Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem Cells Dev. 2011;20:67–75.
De Barros S, Dehez S, Arnaud E, Barreau C, Cazavet A, Perez G, et al. Aging-related decrease of human ASC angiogenic potential is reversed by hypoxia preconditioning through ROS production. Mol Ther. 2013;21:399–408.
Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–53.
Hung SP, Ho JH, Shih YR, Lo T, Lee OK. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. J Orthop Res. 2012;30:260–6.
Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82:2031–7.
Wang DW, Fermor B, Gimble JM, Awad HA, Guilak F. Influence of oxygen on the proliferation and metabolism of adipose derived adult stem cells. J Cell Physiol. 2005;204:184–91.
Packer L, Fuehr K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature. 1977;267:423–5.
Malladi P, Xu Y, Chiou M, Giaccia AJ, Longaker MT. Effect of reduced oxygen tension on chondrogenesis and osteogenesis in adipose-derived mesenchymal cells. Am J Physiol Cell Physiol. 2006;290:C1139–46.
Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–41.
Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220:562–8.
Grayson WL, Zhao F, Izadpanah R, Bunnell B, Ma T. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol. 2006;207:331–9.
Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112:126–35.
Kofoed H, Sjontoft E, Siemssen SO, Olesen HP. Bone marrow circulation after osteotomy. Blood flow, pO2, pCO2, and pressure studied in dogs. Acta Orthop Scand. 1985;56:400–3.
D’Ippolito G, Diabira S, Howard GA, Roos BA, Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–22.
Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14:518–28.
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–90.
Kumar S, Vaidya M. Hypoxia inhibits mesenchymal stem cell proliferation through HIF1alpha-dependent regulation of P27. Mol Cell Biochem. 2016;415:29–38.
Palomaki S, Pietila M, Laitinen S, Pesala J, Sormunen R, Lehenkari P, et al. HIF-1alpha is upregulated in human mesenchymal stem cells. Stem Cells. 2013;31:1902–9.
Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–5.
Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, et al. Role of hypoxia-inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst. 2004;96:946–56.
Yamamoto Y, Fujita M, Tanaka Y, Kojima I, Kanatani Y, Ishihara M, et al. Low oxygen tension enhances proliferation and maintains stemness of adipose tissue-derived stromal cells. Biores Open Access. 2013;2:199–205.
Tsai CC, Su PF, Huang YF, Yew TL, Hung SC. Oct4 and Nanog directly regulate Dnmt1 to maintain self-renewal and undifferentiated state in mesenchymal stem cells. Mol Cell. 2012;47:169–82.
Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–49.
Yang DC, Yang MH, Tsai CC, Huang TF, Chen YH, Hung SC. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS One. 2011;6:e23965.
Acknowledgements
This work was supported by Biomedical Research Institute Grant, Kyungpook National University Hospital (2014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no Conflict of interest.
Ethical statement
This study was approved by the Ethics Committee of the Kyungpook National University Hospital (IRB No. KNUH 2012-10-018).
Rights and permissions
About this article
Cite this article
Kwon, S.Y., Chun, S.Y., Ha, YS. et al. Hypoxia Enhances Cell Properties of Human Mesenchymal Stem Cells. Tissue Eng Regen Med 14, 595–604 (2017). https://doi.org/10.1007/s13770-017-0068-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-017-0068-8