Abstract
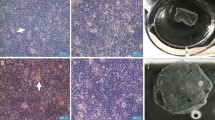
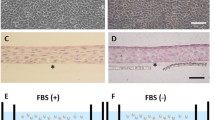
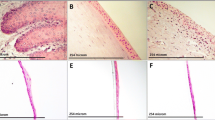
Corneal epithelium regeneration using autologous oral mucosal epithelial cell sheet is a successful new approach in corneal therapy. In the present study, gene expression profiling was performed to characterize engineered cell sheets. Cell sheets were obtained by culturing isolated rabbit oral mucosal epithelial cells on a thermoresponsive cultureware (UpCell®, CellSeed Inc. Japan). H&E staining of cell sheets showed a multistratified epithelium, similar to corneal epithelium. DeltaN-p63 stained positive in the basal cells, indicating that cell sheets have renewal capacity. Microarray analysis of these cell sheets showed that only 160 genes out of 43,000 rabbit probes listed on the microarray chip were identified. We first identified the extracellular matrix group of genes and found that matrix metalloproteinase MMP-1, MMP-3 and MMP-12, known to promote angiogenesis, were down regulated, while MMP-13 and collagen type VIII alpha 1 (COL8A1), proteins involved in wound healing, were up regulated. Tissue inhibitors of metalloproteinase TIMP-1 and TIMP-3, anti-angiogenic factors, were also identified. Gap junction protein A7 (GJA7 or Connexin 45) was found up regulated, indicating that cell sheets have developed well preserved cell-cell interactions. Alcohol dehydrogenase 5 (ADH class III) and aldehyde dehydrogenase (ALDH1A1), involved in protecting the cornea against oxidative stress induced by UV radiation, were also found up regulated. In conclusion, microarray analysis has led us to identify new target molecules and their subsequent biochemical analysis indicated how the composite cell sheets are advantageous to the original isolated cells in terms of the integrity and potency of corneal epithelial grafts without any scaffolds.
Similar content being viewed by others
References
SC Tseng, SY Chen, YC Shen, et al., Critical appraisal of ex vivo expansion of human limbal epithelial stem cells, Curr Mol Med, 10, 841 (2010).
C Dieckmann, R Renner, L Milkova, et al., Regenerative medicine in dermatology: biomaterials, tissue engineering, stem cells, gene transfer and beyond, Exp Dermatol, 19, 697 (2010).
M Krakauer, JD Welder, HK Pandya, et al., Adverse effects of systemic immunosuppression in keratolimbal allograft, J Ophthalmol, 2012, 576712 (2012).
T Nakamura, K Endo, LJ Cooper, et al., The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane, Invest Opthalmol Vis Sci, 44, 106 (2003).
K Nishida, M Yamato, Y Hayashida, et al., Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface, Transplantation, 15, 379 (2004).
C Burillon, L Huot, V Justin, et al., Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency, Invest Ophthalmol Vis Sci, 13, 1325 (2012).
Y Hayashida, K Nishida, M Yamato, et al., Ocular surface reconstruction using autologous rabbit oral mucosal epithelial sheets fabricated ex vivo on a temperature-responsive culture surface, Invest Ophthalmol Vis Sci, 46, 1632 (2005).
Y Barrandon, H Green, Three clonal types of keratinocyte with different capacities for multiplication, Proc Natl Acad Sci USA, 84, 2302 (1987).
I Bortolomai, S Canevari, I Facetti, et al., Tumor initiating cells: development and critical characterization of a model derived from the A431 carcinoma cell line forming spheres in suspension, Cell Cycle, 15, 1194 (2010).
SL Karsten, LC Kudo, R Jackson, et al., Global analysis of gene expression in neural progenitors reveals specific cell-cycle, signaling, and metabolic networks, Dev Biol, 261, 165 (2003).
N Li, S Singh, P Cherukuri, et al., Reciprocal intraepithelial interactions between TP63 and hedgehog signaling regulate quiescence and activation of progenitor elaboration by mammary stem cells, Stem Cells, 26, 1253 (2008).
DW Huang, BT Sherman, RA Lempicki, Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists, Nucleic Acids Res, 37, 1 (2009).
DW Huang, BT Sherman, RA Lempicki, et al., Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources, Nature Protoc, 4, 44 (2009).
H Nagase, JJ Enghild, K Suzuki, et al., Stepwise activation mechanisms of the precursor of matrix metalloproteinase 3 (stromelysin) by proteinases and (4-aminophenyl) mercuric acetate, Biochemistry, 19, 5783 (1990).
VP Saw, E Schmidt, I Offiah, et al., Profibrotic phenotype of conjunctival fibroblasts from mucous membrane pemphigoid, Am J Pathol, 178, 187 (2011).
T Desronvil, D Logan-Wyatt, W Abdrabou, et al., Distribution of COL8A2 and COL8A1 gene variants in Caucasian primary open angle glaucoma patients with thin central corneal thickness, Mol Vis, 29, 2185 (2010).
AK El-Hawary, E Yassin, A Khater, et al., Expression of matrix metalloproteinase-13 and Ki-67 in nonmelanoma skin cancer in Xeroderma Pigmentosum and non-xeroderma Pigmentosum, Am J Dermatopathol, 35, 45 (2013).
GM Gordon, JS Austin, AL Sklar, et al., Comprehensive gene expression profiling and functional analysis of matrix metalloproteinases and TIMPs, and identification of ADAM-10 gene expression, in a corneal model of epithelial resurfacing, J Cell Physiol, 226, 1461 (2011).
SD Galiacy, C Froment, E Mouton-Barbosa, et al., Deeper in the human cornea proteome using nanoLC-Orbitrap MS/MS: An improvement for future studies on cornea homeostasis and pathophysiology, Proteomics, 10, 81 (2011).
E Candi, A Rufini, A Terrinoni, et al., Differential roles of p63 isoforms in epidermal development: selective genetic complementation in p63 null mice, Cell Death Differ, 13, 1037 (2006).
T Matsuura, VK Kawata, H Nagoshi, et al., Regulation of proliferation and differentiation of mouse tooth germ epithelial cells by distinct isoforms of p51/p63, Arch Oral Biol, 57, 1108 (2012).
MI Koster, S Kim, AA Mills, et al., p63 is the molecular switch for initiation of an epithelial stratification program, Genes Dev, 18, 126 (2004).
AV Danilov, D Neupane, AS Nagaraja, et al., DeltaNp63alphamediated induction of epidermal growth factor receptor promotes pancreatic cancer cell growth and chemoresistance, PLoS One, 6, 26815 (2011).
E Thomas, N Zeps, M Cregan, et al., 14-3-3ó (sigma) regulates proliferation and differentiation of multipotent p63-positive cells isolated from human breastmilk, Cell Cycle, 15, 278 (2011).
D Suzuki, M Senoo, Increased p63 phosphorylation marks early transition of epidermal stem cells to progenitors, J Invest Dermatol, 132, 2461 (2012).
MD Westfall, AS Joyner, CE Barbieri, et al., Ultraviolet radiation induces phosphorylation and ubiquitin-mediated degradation of DeltaNp63alpha, Cell Cycle, 4, 710 (2005).
S Merjava, P Liskova, Y Sado, et al., Changes in the localization of collagens IV and VIII in corneas obtained from patients with posterior polymorphous corneal dystrophy, Exp Eye Res, 88, 945 (2009).
U Hopfer, N Fukai, H Hopfer, et al., Targeted disruption of Col8a1 and Col8a2 genes in mice leads to anterior segment abnormalities in the eye, FASEB J, 19, 1232 (2005).
C Zhang, WR Bell, OH Sundin, et al., Immunohistochemistry and electron microscopy of early-onset fuchs corneal dystrophy in three cases with the same L450W COL8A2 mutation, Trans Am Ophthalmol Soc, 104, 85 (2006).
I Loeffler, M Liebisch, G Wolf, et al., Collagen VIII influences epithelial phenotypic changes in experimental diabetic nephropathy, Am J Physiol Renal Physiol, 303, F733 (2012).
JM Sivak, ME Fini, MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology, Prog Retin Eye Res, 21, 1 (2002).
H Nagase, JF Woessner Jr, Matrix metalloproteinases, J Biol Chem, 274, 21491 (1999).
Z Werb, ECM and cell surface proteolysisregulating cellular ecology, Cell, 91, 439 (1997).
JF Woessner, The matrix metalloproteinase family. In: WC Parks, RP Mecham, eds. Matrix Metalloproteinases. Academic Press. San Diego, CA, 1 (1998).
HQ Ye, M Maeda, FS Yu, et al., Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas, Invest Opthalmol Vis Sci, 41, 2894 (2010).
M.C Kenney, M Chwa, A Alba, et al., Localization of TIMP-1, TIMP-2, TIMP-3, gelatinase A and gelatinase B in pathological human corneas, Curr Eye Res, 17, 238 (1998).
R Visse, H Nagase, Matrix metalloproteinases and tissue inhibitorsof metalloproteinases: structure, function and biochemistry, Circ Res, 92, 827 (2003).
MI Cockett, G Murphy, ML Birch, et al., Matrix metalloproteinases and metastatic cancer, Biochem Soc Symp, 63, 295 (1998).
H Sato, M Seiki, Membrane-type matrix metalloproteinases (MTMMPs) in tumor metastasis, J Biochem, 119, 209 (1996).
D Stagos, Y Chen, M Cantore, et al., Corneal aldehyde dehydrogenases: multiple functions and novel nuclear localization, Brain Res Bull, 15, 211 (2010).
JV Jester, Corneal crystallins and the development of cellular transparency, Semin Cell Dev Biol, 19, 82 (2008).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bardag-Gorce, F., Oliva, J., Wood, A. et al. Microarray analysis of oral mucosal epithelial cell sheet. Tissue Eng Regen Med 10, 362–370 (2013). https://doi.org/10.1007/s13770-013-1103-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-013-1103-z