Abstract
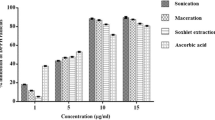
Various extraction solvents and sonication treatment times were compared to determine antioxidant activity and polyphenol composition in mulberry (Morus alba L.) fruits of the Gwasang No. 2 variety. Mulberry fruits were extracted with ethanol, methanol, acidic methanol (0.1% HCl), or acidic ethanol (0.1% HCl) with 0–60-min sonication to measure total phenolic content and antioxidant activity (DPPH radical scavenging activity). Moreover, a high-performance liquid chromatography method to analyze polyphenol compositions in mulberry extracts was developed and levels of common polyphenolic compounds in the different extracts were determined. The effects of different methanol ratios in acidic aqueous methanol (0–100%) on individual polyphenol contents in the extracts after 30 min of sonication were investigated. The total phenolic contents of the extracts using acidic methanol and sonicated for 60 min were significantly higher than phenolic contents upon extraction with other solvents and sonication times (p < 0.001). Interestingly, antioxidant activity of the extracts using acidic ethanol sonicated for 60 min was significantly higher than that of other extracts (p < 0.001). Cyanidin-3-glucoside and 3-rutinoside were the major polyphenol compounds in all mulberry extracts. Cyanidin-3-glucoside and 3-rutinoside concentrations were highest in the acidic methanol extract sonicated for 30 min (15,664 and 19,630 μg/g DW) (p < 0.001). The acidic methanol extract sonicated for 30 min contained significantly higher polyphenolic compound content than that of other extracts (p < 0.001). A decreased methanol ratio (0–80%) in acidic aqueous methanol resulted in chlorogenic acid overestimation in mulberry extracts. Thus, acidic 100% methanol with 30-min sonication is recommended for polyphenol analysis in mulberry.





Similar content being viewed by others
References
Eom SH, Park HJ, Jin CW, Park SM, Kim MJ, Yu CY, Cho DH (2007) Changes of antioxidant activity in Juglans mandshurica Maxim. leaves by far infrared ray irradiation. Korean J Med Crop Sci 15(4):266–270
Hong H, Niu KM, Lee JH, Cho S, Han SG, Kim SK (2016) Characteristics of Chinese chives (Allium tuberosum) fermented by Leuconostoc mesenteroides. Appl Biol Chem 59(3):349–357
Bermundez-Soto MJ, Larrosa M, Garcia-Cantalejo JM, Espín JC, Tomás-Barberan FA, García-Conesa MT (2007) Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J Nutr Biochem 18(4):259–271
Larrosa M, González-Sarrías A, Yáñez-Gascón MJ, Selma MV, Azorín-Ortuño M, Toti S, Tomás-Barberán F, Dolara P, Espín JC (2010) Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem 21(8):717–725
Bae SH, Suh HJ (2007) Antioxidant activities of five different mulberry cultivars in Korea. LWT Food Sci Technol 40(6):955–962
Dugo P, Mondello L, Errante G, Zappia G, Dugo G (2001) Identification of anthocyanins in berries by narrow-bore high-performance liquid chromatography with electrospray ionization detection. J Agric Food Chem 49(8):3987–3992
Narita Y, Inouye K (2013) Degradation kinetics of chlorogenic acid at various pH values and effects of ascorbic acid and epigallocatechin gallate on its stability under alkaline conditions. J Agric Food Chem 61(4):966–972
Ercisli S, Orhan E (2007) Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem 103(4):1380–1384
Aramwit P, Bang N, Srichana T (2010) The properties and stability of anthocyanins in mulberry fruits. Food Res Int 43(4):1093–1097
Du Q, Zheng J, Xu Y (2008) Composition of anthocyanins in mulberry and their antioxidant activity. J Food Compos Anal 21(5):390–395
Bao T, Xu Y, Gowd V, Zhao J, Xie J, Liang W, Chen W (2016) Systematic study on phytochemicals and antioxidant activity of some new and common mulberry cultivars in China. J Funct Foods 25:537–547
Natic MM, Dabic DC, Papetti A, Fotiric Akšic MM, Ognjanov V, Ljubojevic M, Tešic ZL (2015) Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem 171:128–136
Ceymann M, Arrigoni E, Scharer H, Nising AB, Hurrell RF (2012) Identification of apples rich in health-promoting flavan-3-ols and phenolic acids by measuring the polyphenol profile. J Food Compos Anal 26(1–2):128–135
Vieira FGK, Borges GDSC, Copetti C, Pietro PFD, Nunes EDC, Fett R (2011) Phenolic compounds and antioxidant activity of the apple flesh and peel of eleven cultivars grown in Brazil. Sci Hortic 128(3):261–266
Wanyo P, Kaewseejan N, Meeso N, Siriamornpun S (2016) Bioactive compounds and antioxidant properties of different solvent extracts derived from Thai rice by-products. Appl Biol Chem 59(3):373–384
Ludwig IA, Mena P, Calani L, Borges G, Pereira-Caro G, Bresciani L, Rio DD, Lean MEJ, Crozier A (2015) New insights into the bioavailability of red raspberry anthocyanins and ellagitannins. Free Radic Biol Med 89:758–769
Falleh H, Ksouri R, Lucchessi ME, Abdelly C, Magné C (2012) Ultrasound-assisted extraction: effect of extraction time and solvent power on the levels of polyphenols and antioxidant activity of Mesembryanthemum edule L. Aizoaceae shoots. Trop J Pharm Res 11(2):243–249
Ardestani SB, Sahari MA, Barzegar M (2016) Effect of extraction and processing conditions on anthocyanins of barberry. J Food Process Preserv 40(6):1407–1420
Aadil RM, Zeng XA, Han Z, Sun DW (2013) Effects of ultrasound treatments on quality of grapefruit juice. Food Chem 141(3):3201–3206
Sinela A, Rawat N, Mertz C, Achir N, Fulcrand H, Dornier M (2017) Anthocyanins degradation during storage of Hibiscus sabdariffa extract and evolution of its degradation products. Food Chem 214:234–241
Ozela EF, Stringheta PC, Chauca MC (2007) Stability of anthocyanin in spinach vine (Basella rubra) fruits. Cienc Invest Agrar 34(2):115–120
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean government (MSIP) (No. 2016R1C1B1014851). This research was supported by the Chung-Ang University Graduate Research Scholarship in 2017.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, I., Lee, J. Comparison of different extraction solvents and sonication times for characterization of antioxidant activity and polyphenol composition in mulberry (Morus alba L.). Appl Biol Chem 60, 509–517 (2017). https://doi.org/10.1007/s13765-017-0303-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-017-0303-y