Abstract
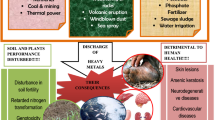
Adsorption of chromium (VI), lead (II) and zinc (II) metal ions from the industrial effluent by tobacco leaves coated with iron oxides was investigated using continuous fixed bed column. The nano-adsorbent material is prepared by chemical synthesis technique capped with iron oxide nanoparticles, and its characteristics are analysed by various surface morphological methods. Breakthrough analysis of the column was examined by varying the adsorbate concentrations, height of the adsorbent bed and rate of flow. The maximum adsorption efficiencies of chromium (VI)–92.26%, lead (II)–75.57% and zinc (II)–89.36% were observed with 5 mL/min of optimum flow rate, bed height of 10 cm and metal ion concentration of 100 mg/L. Kinetic models such as Thomas, Yoon-Nelson and bed depth service time were fitted with best correlation (R2 > 0.95) values in this adsorption process. Overall performance of the column was evaluated by desorption studies, and regeneration of adsorbent in the column was also done by adding concentrated hydrochloric acid (0.3 M).





Similar content being viewed by others
References
Akpen GD, Aho MI, Baba N (2018) Adsorption of cadmium (II) from simulated wastewater using albizia samanpod activated carbon in fixed bed columns. Niger J Technol 37(3):833–840
Alghamdi AA, Al-Odayni AB, Saeed WS, Al-Kahtani A, Alharthi FA, Aouak T (2019) Efficient Adsorption of Lead (II) from aqueous phase solutions using polypyrrole – based activated carbon. Materials 12(12):1–16
Ali A, Saeed K, Mabood F (2016) Removal of chromium (VI) from aqueous medium using chemically modified banana peels as efficient low cost adsorbent. Alex Eng J 55:2933–2942
Amin MT, Alazba AA, Shafiq M (2017) Batch and fixed bed column studies for the biosorption of Cu (II) and Pb(II) by raw and treated date palm leaves and orange peel. Global Nest J 19(3):464–478
Badmus MAO, Audu TOK, Anyata BU (2007) Removal of Lead Ion from Industrial Wastewaters by Activated Carbon Prepared from Periwinkle Shells (Typanotonus fuscatus). Turk J Eng Environ Sci 31:251–263
Bayuo J, Pelig-Ba KB, Abukari MA (2019) Adsorptive removal of chromium(VI) from aqueous solution unto groundnut shell. Appl Water Sci 9:107. https://doi.org/10.1007/s13201-019-0987-8
Biswajit sangha, Tarun kumar naiya, Ashim kumar bhattacharya, sudipkumar das, (2011) Cr (VI) ions removal from aqueous solutions using natural adsorbents – FTIR studies. J Environ Protect 2:729–735. https://doi.org/10.4236/jep.2011.26084
Biswas S, Mishra U (2015) Continious fixed bed column study and adsorption modeling: removal of lead ion from aqueous solution by charcoal originated from chemical carbonization of rubber wood sawdust. J Chem. https://doi.org/10.1155/2015/907379
Boudrahem F, Aissani – Benissad F, Soualah A, (2011) Adsorption of Lead (II) from aqueous solution by using leaves of date trees as an adsorbent. J Chem Eng Data 56(05):1804–1812
Boycheva S, Zgureva D, Miteva S, Marinov I, Behunova DM, Trendafilova I, Popova M, Vaclavikoda M (2020) Studies on the potential of Nanomodified and metal oxide modified coal fly ash zeolites for adsorption of heavy metals and catalyic degradation of organics for waste water recovery. Processes 8:778
Çekim M, Yildiz S, Dere T (2015) Biosorption of copper from synthetic waters by using tobacco leaf: Equilibrium, kinetic and thermodynamic tests. J Environ Eng Lands Manag 23(03):172–182. https://doi.org/10.3846/16486897.2015.1050398
Chen Yunnen Wu, Ye LC, Lin G, Jinxian N, Rushan R (2017) Continious fixed bed column study and adsorption modellig: Removal of arsenate and arsenic in aqueous solution by organic modified spent grains. Polish J Environ Stud 26(4):1847–1854
Christian Taty-Costodes V, Fauduet H, Porte C, Ho Y-S (2005) Removal of lead (II) ions from synthetic and real effluents using immobilized pinus sylvestris saw dust: adsorption on a fixed bed column. J Hazard Mater B123:135–144
Dave PN, Chopda LV (2014) Application of iron oxide nanomaterials for the removal of heavy metals. J Nanotechnol. https://doi.org/10.1155/2014/398569
Farghali AA, Bahgat M, Enaiet Allah A, Khedr MH (2013) Adsorption of Pb(II) ions from aqueous solutions using copper oxide nanostructures. Beni- suef Univ J Basic Appl Sci 2(2):61–71. https://doi.org/10.1016/j.bjbas.2013.01.001
Fauzia S, Aziz H, Dahlan D, Zein R (1889) The feasibility of sago bark (metroxylon sagu) in Cu (II) removal: batch and fixed bed column evaluation. Rasayan J Chem 12(4):1900
Frutos I, García-Delgado C, Gárate A (2016) Biosorption of heavy metals by organic carbon from spent mushroom substrates and their raw materials. Int J Environ Sci Technol 13:2713–2720. https://doi.org/10.1007/s13762-016-1100-6
Gopal N, Asaithambi M, Sivakumar P, Sivakumar V (2016) Continious fixed bed adsorption studies of rhodamine – B dye using polymer bound adsorbent. Indian J Chem Technol 23:53–58
Hala ahmed Hegazi (2013) Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents. HRBC J 9:276–282. https://doi.org/10.1016/j.hbrcj.2013.08.004
Hasfalina CM, Maryam CA, Luqman RZ, Rashid M (2012) Adsorption of Copper (II) from aqueous medium in fixed bed column by kenaf fibres. APCBEE Procedia 3:255–263
Hong J, Xie J, Mirshahghassemi S, Lead J (2020) Metal (Cu, Cr, Ni, Pb) removal from environmentally relevant waters using polyvinylpyrrolidone – coated magnetic nanoparticles. RSC Adv 10:3266–3276. https://doi.org/10.1039/C9RA10104G
Igberase E, Osifo P, Ofomaja A (2017) The adsorption of Pb, Zn, Cu, Ni and Cd by modified ligand in a single component aqueous solution: equilibrium, kinetic, thermodynamic and desorption studies. Int J Anal Chem ID. https://doi.org/10.1155/2017/6150209
Ilankoon N (2014) Use of iron oxide magnetic nano adsorbents for Cr(VI) removal from aqueous solutions: a review. Int J Eng Res Appl 4(10):55–63
Jain M, Kumar Garg V, Kadirvelu K (2013) Removal of Ni (II) from aqueous system by chemically modified sunflower biomass. Desalination Water Treat 52:5681–5695
Jiao C, Cheng Y, Fan W, Li J (2012) Synthesis of agar – stabilized nanoscale zero valent iron particles and removal study of hexavalent chromium. Int J Environ Sci Technol 12:1603–1612
Kalaivani SS, Vidhyadevi T, Murugesan A, Thiruvengadaravi KV, Anuradha D, Sivanesan S (2015) The use of new modified poly (acrylamide) chelating resin with pendent benzothiazole groups containing donor atoms in the removal of heavy metal ions from aqueous solutions. Water Resour India 5:21–35
Kalavathy Karthikeyan MH, Rajgopal T, Miranda LR (2007) Kinetic and isotherm studies of Cu (II) adsorption onto HPO-activated rubber wood sawdust. J Coll Interf Sci 292(2):354–362
Kara A, Üstün GE, Akal Solmaz SK, Demirbel E (2013) Removal of Pb (II) ions in fixed bed column from electro plating wastewater of bursa, an industrial city in turkey. J Chem 953968:1–7
Karunarathne HDSS, Amarasinghe BMWPK (2013) Fixed bed adsorption column studies for the removal of aqueous phenol from activated carbon prepared from sugarcane bagasse. Energy Procedia 34:83–90
Koohzad E, Jafari D, Esmaeili H (2019) Adsorption of lead and arsenic ions from aqueous solution by activated carbon prepared from tamarix leaves. Chem Europe 04(42):12356–12367
Kumar U, Acharya J (2013) Fixed bed column study for the removal of lead from aquatic environment by NRCH. Res J Recent Sci 2(1):9–12
Lalitha LM, Mariraj Mohan S (2018) Performance evaluation of multibed adsorbent on removal of hexavalent chromium through various kinetic models. J Environ Eng Landsc Manag 26(4):285–298
Li Q, Zhai W, Zhang W, Wang M, Zhou J (2006) Kinetic studies of adsorption of Pb (II), Cr (III) and Cu (II) from aqueous solution by sawdust and modified peanut husk. J Hazard Mater 141:163–167
Li N, Ren J, Zaho L, Wang Z (2013) Fixed bed adsorption study on phosphate removal using Nanosized FeOOH- modified anion resin. J Nanomater 736275:1–5
Luisa mayra vera, Daniel bermejo, Maria fernanda uguna, Marittza flores, Nancy garcia and Enrique Gonzalez, (2018) Fixed bed column modelling of lead (II) and cadmium (II) ions biosorption on sugarcane bagasse. Environ Eng Res. https://doi.org/10.4491/eer.2018.042
Mahmoud AM, Ibrahim FA, Shaban SA, Youssef NA (2015) Adsorption of heavy metal ion from aqueous solution by nickel oxide nano catalyst prepared by different methods. Egypt J Petrol 24:27–35
Mahmud Huq HNME, Binti Yahya AO (2016) The removal of heavy metal ions from wastewater/aqueous solution using polypyrrole-based adsorbents: a review. RSC Adv 06:14778–14791
Marzbali MH, Esmaieli M (2017) Fixed bed adsorption of tetracycline on a mesoporous activated carbon: experimental study and neuro-fuzzy modelling. J Appl Res Technol 15:454–463
Mohd Azmier Ahmad (2014) Kinetic, equilibrium and thermodynamic studies of synthetic dye removal using pomegranate peel activated carbon prepared by microwave – induced KOH activation. Water Resour Indus 06:18–35
Mulani K, Daniels S, Rajdeo K, Tambe S, Chavan N (2013) Adsorption of chromium (VI) from aqueous solutions by coffee polyphenol – formaldehyde/acetaldehyde resins. J Polym ID. https://doi.org/10.1155/2013/798368
Naddafi K, Nabizadeh R, Saeedi R, Mahvi AH, Vaezi F, Yaghmaeian K, Ghasri A, Nazmara S (2007) Biosorption of lead (II) and Cadmium (II) by protonated sargassum glaucescens biomass in a continious packed bed column. J Hazard Mater 147:785–791
Naik AR, Gawande SM (2015) Management of tendu leaf in Solapur city. Int Res J Eng Technol 02(03):1538–1545
Narasimha Rao C, Subbarayudu K, Vijaya Y, Venkata subbiah M (2014) Adsorption of Ni (II) from aqueous solution by activated carbons derived from tobacco stem. Desal Water Treat 10(1080/19443994):910837
Narges samadani langeroodi, Zhaleh farhadravesh, Aliakbar dehno khalaji, (2018) Optimization of adsorption parameters for Fe (III) ions removal from aqueous solutions by transition metal oxide nanocomposite. Green Chem Lett Rev 11(4):404–413. https://doi.org/10.1080/17518253.2018.1526329
Ogunleye OO, Ajala MA, Agarry SE (2014) Evaluation of biosorptive capacity of banana (Musa paradisiaca) stalk for lead (II) removal from aqueous solution. J Environ Protect 05:1451–1465
Ouyang D, Zhuo Y, Liang Hu, Zeng Q, Yuehua Hu, He Z (2019) Research on the adsorption behaviour of heavy metal ions by porous material prepared with silicate tailings. Minerals 9:291. https://doi.org/10.3390/min9050291
Pandey PK, Sharma SK (2017) Removal of Cr (VI) and Pb (II) from wastewater by ZeoliteNax in fixed bed column. Water Conserv Sci Eng. https://doi.org/10.1007/s41101-017-0026-2
Patel H (2019) Fixed bed column adsorption study: a comprehensive review. Appl Water Sci 9(45):1–17
Piao Xu, Zeng GM, Huang DL, Feng CL, Shuang Hu, Zhao MH, Lai C, Wei Z, Huang C, Xie GX, Liu ZF (2012) Use of iron oxide nano materials in wastewater treatment: a review. Sci Total Environ 424:1–10
Priya AK, Nagan S, Nithya M, Priyanka PM, Rajeswari M (2016) Assessment of tendu leaf refuses for the heavy metal removal from electroplating effluent. J Pure Appl Microbiol 10(1):585–591
Qin F, Wen Q, Shan XQ, Xie YN (2015) Mechanisms of competitive adsorption of Pb, Cu and Cd on peat. Environ Pollut 144:669–680
Renu MA, Singh K, Gupta R, Dohare RK (2020) Continious fixed bed adsorption of heavy metals using biodegradable adsorbent: Modelling and experimental study. J Environ Eng 146(2):04019110
Saadi Z, Saadi R, Fazaeli R (2013) Fixed-bed adsorption dynamics of Pb (II) adsorption from aqueous solution using nanostructured γ-alumina. J Nanostruct Chem. https://doi.org/10.1186/2193-8865-3-48
Saebra AB, Duran N (2015) Nanotechnology of metal oxide nanoparticles. Meals 5(2):934–975. https://doi.org/10.3390/met5020934
Sánchez-Machado DI, López-Cervantes J, Correa-Murrieta MA, Sánchez-Duarte RG (2016) Modelling of breakthrough curves for aqueous iron (III) adsorption on chitosan –sodium tripolyphosphate. Water Sci Technol 74(10):2297–2304. https://doi.org/10.2166/wst.2016.409
Santhosh C, Nivetha R, Kollu P (2017) Removal of cationic and anionic heavy metals from water by 1D and 2D-carbon structures decorated with magnetic nanoparticles. Sci Rep 7:14107. https://doi.org/10.1038/s41598-017-14461-2
Senthil Kumar P, Ramalingam S, Sathyaselvabala V, Dinesh Kirupha S, Murugesan A, Sivanesan S (2012) Removal of cadmium (II) from aqueous solution by agricultural waste cashew nut shel. Korean J Chem Eng 29(6):756–768
Singh TP, Ghosh S, Majumder CB (2016) Adsorption of fluoride from industrial wastewater in fixed bed column using java plum. Asian J Pharm Cliniical Res 9(3):320–327
Solgi M, Tabil LG, Wilson LD (2020) Modified biopolymer adsorbents for column treatment of sulfate species in saline aquifers. Materials 13:2408. https://doi.org/10.3390/ma13102408
Sukumar C, Janaki C, Vijayaraghavan K, Kamala Kannan S, Shanthi K (2016) Removal of Cr (VI) using co-immobilized activated carbon and Bacillus subtilis: fixed bed column study. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-016-1203-2
Vidhyadevi T, Murugesan A, Kalaivani SS, Premkumar MP, Vinothkumar V, Ravikumar L (2014) Evaluation of equilibrium, kinetic, and thermodynamic parameters for adsorption of Cd2+ ion and methyl red dye onto amorphous poly(azomethinethioamide) resin. Desal Water Treat 52:3477–3488
Vishnu manirathan, Niharika gupta, Raj mohan balakrishnan, Keyur raval, (2020) Batch and continuous studies on the removal of heavy metals from aqueous solution using biosynthesised melanin-coated PVDF membranes. Environ Sci Pollut Res 27:24723–24737. https://doi.org/10.1007/s11356-019-06310-8
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Fixed bed dynamic column adsorption study of methylene blue (MB) onto pine cone. Desalination Water Treat 55(04)1026–1039
Yahya MD, Aliyu AS, Obayomi KS, Olugbenga AG, Abdullahi UB (2020) Column adsorption study for the removal of chromium and manganese from electroplating wastewater using cashew nutshell adsorbent. Chem Eng 7:1748470. https://doi.org/10.1080/23311916.2020.1748470
Yakout SME, Abdeltawab AA, Elhindi K, Askalany A (2018) Uranium dynamic adsorption breakthrough curve onto rice straw based activated carbon using bed depth service time model. BioResources 13(4):9143–9157
Yang J, Hou B, Wang J, Tian B, Bi J, Wang N, Li X, Huang X (2019) Nanomaterials for the removal of heavy metals from wastewater. Nanomaterials 9:424. https://doi.org/10.3390/nano9030424
Yarkandi NY (2014) Removal of lead (II) from waste water by adsorption. Int J Curr Microbiol Appl Sci 3(4):207–228
Yildiz I, Sizirici B (2019) Iron oxide coated gravel fixed bed column study performance to remove mixed metals from landfill lechate. E3S web of conferences, 122: 01002. https://doi.org/10.1051/e3sconf/201912201002
Yogeshwaran V, Priya AK (2019) Removal of heavy metals using nano-particles – a review. Indian J Environ Prot 39(1):17–21
Zhang Y, Bing Wu, Hui Xu, Liu H, Wang M, He Y, Pan B (2016) Nanomaterias – enabled water and wastewater treatment. NanoImpact 3(4):22–39
Zhang X, Guo S, Liu J, Zhang Z, Song K, Tan C, Li H (2019) A study on the removal of copper (II) from aqueous solution using lime sand bricks. Appl Sci 9(4):670. https://doi.org/10.3390/app9040670
Acknowledgement
The authors need to acknowledge the research scholars of Nanotechnology Department from Government College of Technology, Coimbatore, for providing valuable support to synthesize the nanoparticles and its characterization study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: S Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Venkatraman, Y., Priya, A.K. Removal of heavy metal ion concentrations from the wastewater using tobacco leaves coated with iron oxide nanoparticles. Int. J. Environ. Sci. Technol. 19, 2721–2736 (2022). https://doi.org/10.1007/s13762-021-03202-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-021-03202-8