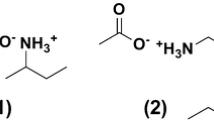
Abstract
Microwave-assisted tetrabutyl ammonium-impregnated sulphate-crosslinked chitosan was synthesized for enhanced adsorption of hexavalent chromium. The adsorbent obtained was extensively characterized using Fourier transform infrared, X-ray diffraction, scanning electron microscopy and energy-dispersive X-ray studies. Various isotherm models such as Langmuir, Freundlich and Dubinin–Radushkevich were studied to comprehend the adsorption mechanism of hexavalent chromium by the adsorbent. Maximum adsorption capacity of 225.9 mg g−1 was observed at pH 3.0 in accordance with Langmuir isotherm model. The sorption kinetics and thermodynamic studies revealed that adsorption of hexavalent chromium followed pseudo-second-order kinetics with exothermic and spontaneous behaviour. A column packed with 1 g of adsorbent was found to give complete adsorption of Cr(VI) up to 900 mL of 200 mg L−1 solution which discerns the applicability of the adsorbent material for higher sample volumes in column studies. The effective adsorption results were obtained due to both ion exchange and ion pair interaction of adsorbent with hexavalent chromium. Greener aspect of overall adsorption was regeneration of the adsorbent which was carried out using sodium hydroxide solution. In the present study, the regenerated adsorbent was effectively reused up to ten adsorption–desorption cycles with no loss in adsorption efficiency.










Similar content being viewed by others
References
Aydin YA, Aksoy ND (2009) Adsorption of chromium on chitosan: optimization, kinetics and thermodynamics. Chem Eng J 151:188–194
Bhatnagar A, Sillanpaa M (2009) Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—a short review. Adv Colloid Interface Sci 152:26–38
Bulut E, Ozacar M, Sengil IA (2008) Adsorption of malachite green onto bentonite: equilibrium and kinetic studies and process design. Microporous Mesoporous Mater 115:234–246
Crini G, Peindy HN, Gimbert F, Robert C (2007) Removal of CI basic green 4 (Malachite Green) from aqueous solution by adsorption using cyclodextrin based adsorbent: kinetic and equilibrium studies. Sep Purif Technol 53:97–110
Debnath S, Maity A, Pillay K (2014) Magnetic chitosan-go nanocomposite: synthesis, characterization and batch adsorber design for Cr(VI) removal. J Environ Chem Eng 2:963–973
Devi MG, Al-Hashmi ZSS, Sekhar GC (2012) Treatment of vegetable oil mill effluent using crab shell chitosan as adsorbent. Int J Environ Sci Technol 9(4):713–718
Donia AM, Atia AA, El-Boraey HA, Mabrouk D (2006) Uptake studies of copper(II) on glycidyl methacrylate chelating resin containing Fe2O3 particles. Sep Purif Technol 49:64–70
Dubinin MM, Radushkevich LV (1947) The equation of the characteristic curve of the activated charcoal. Proc Acad Sci USSR Phys Chem Sect 55:331–337
Elwakeel KZ (2014) Removal of arsenate from aqueous media by magnetic chitosan resin immobilized with molybdate oxoanions. Int J Environ Sci Technol 11(4):1051–1062
Freundlich HMF (1906) Over the adsorption in solution. Z Phys Chem 57:385–470
Hayes BL (2004) Recent advances in microwave-assisted synthesis. Aldrichimica Acta 37(2):66–76
Ho YS (2006) Review of second-order models for adsorption systems. J Hazard Mater B 136:681–689
Houk KN, Leach AG, Kim SP, Zhang X (2003) Binding affinities of host–guest, protein–ligand, and protein-transition-state complexes. Angew Chem Int Ed 42:4872–4897
Hu XJ, Wang JS, Liu YG, Li X, Zeng GM, Bao ZL, Zeng XX, Chen AW, Long F (2011) Adsorption of chromium(VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: isotherms, kinetics and thermodynamics. J Hazard Mater 185:306–314
Kahu S, Saravanan D, Jugade R (2014) Effective detoxification of hexavalent chromium using sulfate-crosslinked chitosan. Water Sci Technol 70:2047–2055
Kalidhasan S, Gupta PA, Cholleti VR, Kumar ASK, Rajesh V, Rajesh N (2012) Microwave assisted solvent free green preparation and physicochemical characterization of surfactant-anchored cellulose and its relevance toward the effective adsorption of chromium. J Colloid Interface Sci 372:88–98
Katz SA, Salem H (2006) The toxicology of chromium with respect to its chemical speciation: a review. J Appl Toxicol 13:217–224
Kumar ASK, Rajesh N (2013) Exploring the interesting interaction between grapheme oxide, Aliquat-336 (a room temperature ionic liquid) and chromium(VI) for wastewater treatment. RSC Adv 3:2697–2709
Kumar ASK, Gupta T, Kakan SS, Kalidhasan S, Manasi S, Rajesh V, Rajesh N (2012) Effective adsorption of hexavalent chromium through a three centre (3c) co-operative interaction with an ionic liquid and biopolymer. J Hazard Mater 239–240:213–224
Kumar ASK, Kumar CU, Rajesh V, Rajesh N (2014a) Microwave assisted preparation of n-butylacrylate grafted chitosan and its application for Cr(VI) adsorption. Int J Biol Macromol 66:135–143
Kumar ASK, Sharma S, Reddy RS, Barathi M, Rajesh N (2014b) Comprehending the interaction between chitosan and ionic liquid for the adsorption of palladium. Int J Biol Macromol 72:633–663
Kumar ASK, Jiang SJ, Tseng WL (2015) Effective adsorption of chromium(VI)/Cr(III) from aqueous solution using ionic liquid functionalized multiwalled carbon nanotubes as a super sorbent. J Mater Chem A. doi:10.1039/c4ta06948j
Kyzas GZ, Bikiaris DN (2015) Recent modifications of chitosan for adsorption applicatons: a critical and schematic review. Mar Drugs 13:312–337
Lagergren S (1898) Zur theorie der sogennanten adsorption geloster Stoffe. K. Sven. Vetenskapsakad. Handlingar 24:1–39
Langmuir I (1918) The adsorption of gases on plane surface of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lewinsky AA (2007) Hazardous materials and wastewater: treatment, removal and analysis. Nova Publishers, New York
Mayyas MA (2012) Properties of chitosan nanoparticles formed using sulphate anions as crosslinking bridges. Am J Appl Sci 9:1091–1100
Mendham VJ, Denny RC, Barnes JD, Thomas MJK (2002) Vogel’s textbook of quantitative chemical analysis, 6th edn. Pearson Education, Singapore
Rajesh N, Krishna Kumar AS, Kalidhasan S, Rajesh V (2011) Trialkylamine impregnated macroporous polymeric sorbent for the effective removal of chromium from industrial wastewater. Chem Eng Data 56:2295–2304
Shekhawat A, Kahu S, Saravanan D, Jugade R (2015) Synergistic behavior of ionic liquid impregnated sulphate crosslinked chitosan towards adsorption of Cr(VI). Int J Biol Macromol 80:615–626
Shekhawat A, Kahu S, Saravanan D, Jugade R (2016) Assimilation of chitin with tin for defluoridation of water. RSC Adv. doi:10.1039/C6RA00014B
Tirgar A, Golbabaei F, Hamedi J, Nourijelyani K, Shahtaheri SJ, Moosavi SR (2006) Removal of airborne hexavalent chromium mist using chitosan gel beads as a new control approach. Int J Environ Sci Technol 3(3):305–313
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div Am Soc Civil Eng 89:3–60
Yan Z, Haijia S, Tianwei T (2007) Adsorption behaviors of the aminated chitosan adsorbent. Korean J Chem Eng 24:1047–1052
Zhu L, Liu Y, Chen J (2009) Synthesis of N-methylimidazolium functionalized strongly basic anion exchange resins for adsorption of Cr(VI). Ind Eng Chem Res 48:3261–3267
Acknowledgments
The authors are thankful to RTM Nagpur University for University Research Project No. Dev/1336 (2014–16).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kahu, S., Shekhawat, A., Saravanan, D. et al. Ionic solid-impregnated sulphate-crosslinked chitosan for effective adsorption of hexavalent chromium from effluents. Int. J. Environ. Sci. Technol. 13, 2269–2282 (2016). https://doi.org/10.1007/s13762-016-1059-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1059-3