Abstract
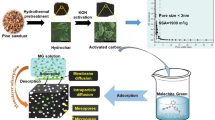
The agricultural wastes like date palm seeds to be a suitable precursor for the preparation of porous carbon has been explored in the present work, utilizing phosphoric acid as the activating agent. The experimental methods reported in literature were chosen with certain modification in order to simplify the process. The process optimization was performed using the popular response surface methodology adopting a Box-Behnken design. Process optimization was performed to maximize the porous carbon Brunauer–Emmett–Teller (BET) surface area and the methylene blue (MB) adsorption capacity, with the process variables being the activation temperature, impregnation ratio (IR) and the activation time. The textural characteristics were assessed based on nitrogen adsorption isotherms, scanning electron microscopy, while the adsorption capacity was estimated using the MB adsorption. The optimized experimental conditions were identified to be an activation temperature of 500 °C, IR of 3.1 and activation time of 71.4 min, with the resultant porous carbon having BET surface area of 846.7 m2/g and MB adsorption capacity of 445.7 mg/g. The popular Langmuir and Freundlich adsorption isotherm models were tested, and a maximum monolayer adsorption capacity of the MB was estimated to be 345 mg/g, which compares with the highest of MB reported in literature, evidencing the suitability of the porous carbon for adsorption of macro-molecular compounds.






Similar content being viewed by others
References
Ahmadpour A, Do DD (1996) The preparation of active carbons from coal by chemical and physical activation. Carbon 34(4):471–479
Al-Qaessi F, Abu-Farah L (2010) Activated carbon production from date stones using phosphoric acid. Energy Sour Part A 32(14):1316–1325
Attia AA, Girgis BS, Fathy NA (2008) Removal of methylene blue by carbons derived from peach stones by H3PO4 activation: batch and column studies. Dyes Pigment 76(1):282–289
Azevedo DCS, Araújo JCS, Bastos-Neto M, Torres AEB, Jaguaribe EF, Cavalcante CL (2007) Microporous activated carbon prepared from coconut shells using chemical activation with zinc chloride. Microporous Mesoporous Mater 100(1–3):361–364
Baçaoui A, Yaacoubi A, Dahbi A, Bennouna C, Phan Tan Luu R, Maldonado-Hodar FJ, Rivera-Utrilla J, Moreno-Castilla C (2001) Optimization of conditions for the preparation of activated carbons from olive-waste cakes. Carbon 39(3):425–432
Banat F, Al-Asheh S, Al-Makhadmeh L (2003) Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Process Biochem 39(2):193–202
Bansal RC, Goyal M (2005) Activated carbon adsorption. Taylor & Francis, Boca Raton
Baquero MC, Giraldo L, Moreno JC, Suarez G, Nez-Alonso A, Tascon JMD (2003) Activated carbons by pyrolysis of coffee bean husks in presence of phosphoric acid. J Anal Appl Pyrol 70(2):779–784
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76(5):965–977
Caturla F, Molina-Sabio M, Rodriguez-Reinoso F (1991) Preparation of activated carbon by chemical activation with ZnCl2. Carbon 29(7):999–1007
Chan LS, Cheung WH, McKay G (2008) Adsorption of acid dyes by bamboo derived activated carbon. Desalination 218(1–3):304–312
Demiral H, Demiral I, Tumsek F, Karabacakoglu B (2008) Adsorption of chromium(VI) from aqueous solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chem Eng J 144(2):188–196
Diao Y, Walawender WP, Fan LT (2002) Activated carbons prepared from phosphoric acid activation of grain sorghum. Bioresour Technol 81(1):45–52
El-Naas MH, Al-Zuhair S, Alhaija MA (2010a) Reduction of COD in refinery wastewater through adsorption on date-pit activated carbon. J Hazard Mater 173(1–3):750–757
El-Naas MH, Al-Zuhair S, Alhaija MA (2010b) Removal of phenol from petroleum refinery wastewater through adsorption on date-pit activated carbon. Chem Eng J 162(3):997–1005
Fierro V, Muñiz G, Basta AH, El-Saied H, Celzard A (2010) Rice straw as precursor of activated carbons: activation with ortho-phosphoric acid. J Hazard Mater 181(1–3):27–34
Garg VK, Amita M, Kumar R, Gupta R (2004) Basic dye (methylene blue) removal from simulated wastewater by adsorption using Indian Rosewood sawdust: a timber industry waste. Dyes Pigment 63(3):243–250
Girgis BS, El-Hendawy ANA (2002) Porosity development in activated carbons obtained from date pits under chemical activation with phosphoric acid. Microporous Mesoporous Mater 52(2):105–117
Gönen F, Aksu Z (2008) Use of response surface methodology (RSM) in the evaluation of growth and copper(II) bioaccumulation properties of Candida utilis in molasses medium. J Hazard Mater 154(1–3):731–738
Guo Y, Rockstraw DA (2007) Physicochemical properties of carbons prepared from pecan shell by phosphoric acid activation. Bioresour Technol 98(8):1513–1521
Gurrath M, Kuretzky T, Boehm HP, Okhlopkova LB, Lisitsyn AS, Likholobov VA (2000) Palladium catalysts on activated carbon supports: influence of reduction temperature, origin of the support and pretreatments of the carbon surface. Carbon 38(8):1241–1255
Haimour NM, Emeish S (2006) Utilization of date stones for production of activated carbon using phosphoric acid. Waste Manag 26(6):651–660
Hameed BH, Salman JM, Ahmad AL (2009) Adsorption isotherm and kinetic modeling of 2,4-D pesticide on activated carbon derived from date stones. J Hazard Mater 163(1):121–126
Huiping L, Guoqun Z, Shanting N, Yiguo L (2007) Technologic parameter optimization of gas quenching process using response surface method. Comput Mat Sci 38(4):561–570
Kannan N, Sundaram MM (2001) Kinetics and mechanism of removal of methylene blue by adsorption on various carbons: a comparative study. Dyes Pigment 51(1):25–40
László K, Bóta A, Nagyu LG (1997) Characterization of activated carbons from waste materials by adsorption from aqueous solutions. Carbon 35(5):593–598
Lim WC, Srinivasakannan C, Balasubramanian N (2010) Activation of palm shells by phosphoric acid impregnation for high yielding activated carbon. J Anal Appl Pyrol 88(2):181–186
Lyubchik SB, Benoit R, Beguin F (2002) Influence of chemical modification of anthracite on the porosity of the resulting activated carbons. Carbon 40(8):1287–1294
Malik R, Ramteke DS, Wate SR (2007) Adsorption of malachite green on groundnut shell waste based powdered activated carbon. Waste Manag 27(9):1129–1138
Mazyck DW, Cannon FS (2000) Overcoming calcium catalysis during the thermal reactivation of granular activated carbon: part I. Steam-curing plus ramped-temperature N2 treatment. Carbon 38(13):1785–1799
Prahas D, Kartika Y, Indraswati N, Ismadji S (2008) Activated carbon from jackfruit peel waste by H3PO4 chemical activation: pore structure and surface chemistry characterization. Chem Eng J 140(1–3):32–42
Puziy AM, Poddubnaya OI, Martinez-Alonso A, Suarez-Garcia F, Tascon JMD (2002) Characterization of synthetic carbons activated with phosphoric acid. Appl Surf Sci 200(1–4):196–202
Rodríguez-Reinoso F, Molina-Sabio M, Gonzalez MT (1995) The use of steam and CO2 as activating agents in the preparation of activated carbons. Carbon 33(1):15–23
Salman JM, Njoku VO, Hameed BH (2011) Bentazon and carbofuran adsorption onto date seed activated carbon: kinetics and equilibrium. Chem Eng J 173(2):361–368
Srinivasakannan C, Zailani Abu Bakar M (2004) Production of activated carbon from rubber wood sawdust. Biomass Bioenergy 27(1):89–96
Stavropoulos GG, Zabaniotou AA (2005) Production and characterization of activated carbons from olive-seed waste residue. Microporous Mesoporous Mater 82(1–2):79–85
Taghdiri M, Zamani N (2013) Hexamine adsorption study on activated carbon from aqueous solutions for application in treatment of hexamine industrial wastewater. Int J Environ Sci Technol 10(1):19–26
Tan IAW, Ahmad AL, Hameed BH (2008a) Optimization of preparation conditions for activated carbons from coconut husk using response surface methodology. Chem Eng J 137(3):462–470
Tan IAW, Ahmad AL, Hameed BH (2008b) Preparation of activated carbon from coconut husk: optimization study on removal of 2,4,6-trichlorophenol using response surface methodology. J Hazard Mater 153(1–2):709–717
Teng H, Yeh TS, Hsu LY (1998) Preparation of activated carbon from bituminous coal with phosphoric acid activation. Carbon 36(9):1387–1395
Valdés H, Sánchez-Polo M, Rivera-Utrilla J, Zaror CA (2002) Effect of ozone treatment on surface properties of activated carbon. Langmuir 18(6):2111–2116
Walker GM, Weatherley LR (1998) Fixed bed adsorption of acid dyes onto activated carbon. Environ Pollut 99(1):133–136
Wang S, Zhu ZH, Coomes A, Haghseresht F, Lu GQ (2005) The physical and surface chemical characteristics of activated carbons and the adsorption of methylene blue from wastewater. J Colloid Interface Sci 284(2):440–446
Behera SK, Oh SY, Park HS (2012) Sorptive removal of ibuprofen from water using selected soil minerals and activated carbon. Int J Environ Sci Technol 9(1):85–94
Zhang J, Lin L, Chen T, Zhang J, Liu S, Li Z, Ouyang P (2009) Dissolution of microcrystalline cellulose in phosphoric acid-molecular changes and kinetics. Molecules 14(12):5027–5041
Zuo S, Yang J, Liu J, Cai X (2005) Significance of the carbonization of volatile pyrolytic products on the properties of activated carbons from phosphoric acid activation of lignocellulosic material. Fuel Process Technol 90(7–8):994–1001
Acknowledgments
The authors wish to acknowledge The Petroleum Institute for giving financial support to work on porous carbon for Hydrogen Storage (RIFP13/12).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, K.S.K., Al Shoaibi, A. & Srinivasakannan, C. Preparation of porous carbon from date palm seeds and process optimization. Int. J. Environ. Sci. Technol. 12, 959–966 (2015). https://doi.org/10.1007/s13762-013-0468-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-013-0468-9