Abstract
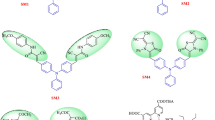
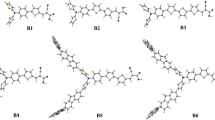
Herein, we report the design and synthesis of six symmetric metal-free organic sensitizers (llyu1a, llyu1b, llyu1c, llyu2a, llyu2b, and llyu2c) based on fluorene or dimethyl fluorene donors core carrying double acceptors. All these dyes were characterized using UV–Vis, ESI-MS, and 1HNMR. To study the influence of π-bridges on total solar-to-electric conversion efficiency (%η) for DSSCs, three different π-bridges thiophene, furane, or benzene were introduced into the sensitizers. Their device performances were studied and showed a distinctive difference in efficiency with a maximum of PCE of 2.35% (Jsc = 5.63 mA cm − 2, VOC = 0.60 V and FF = 70.00%) for dye llyu1a. Density functional theory (DFT) and time-dependent density functional theory calculations were used to probe the relationship between chemical structure, photophysical, and photoelectrochemical properties. DFT studies showed that the dihedral angle between thiophene and donor is 26.6°, indicating that the dyes bearing thiophene π-bridge possess more efficient photoexcitation compared to dyes bearing benzene π-bridge (36.6° for both llyu2c and llyu1c) and less aggregation than dyes bearing furane π-bridge (0° for llyu1b and llyu2b). This new finding of influence of π-bridges on total solar-to-electric conversion efficiency would open the door for the molecular engineering of better light harvesting and more efficient metal-free organic sensitizers for DSSCs.







Similar content being viewed by others
References
B. O’Regan, M. Grätzel, Nature 353, 737 (1991)
M. Dadkhah, M. Salavati-Niasari, Mat. Sci. Semicond. Process 20, 41 (2014)
H. Teymourinia, M. Salavati-Niasari, O. Amiri, M. Farangi, J. Mol. Liq. 251, 267 (2018)
O. Amiri, M. Salavati-Niasari, N. Mir, F. Beshkar, F. Ansari, Renew. Energy 125, 590 (2018)
M.S. Morassaei, A. Salehabadi, A. Akbari, S.H. Tavassoli, M. Salavati-Niasari, J. Alloy Compd. 769, 732 (2018)
N. Mir, M. Salavati-Niasari, F. Davar, Chem. Eng. J. 181–182, 779 (2012)
N. Mir, M. Salavati-Niasari, Mater. Res. Bull. 48, 1660 (2013)
M. Noshin Mir, Salavati-Niasari. Sol. Energy 86, 3397 (2012)
M. Grätzel, Acc. Chem. Res. 42, 1788 (2009)
R. Rattanawan, V. Promarak, T. Sudyoadsuk, S. Namuangrukc, N. Kungwan, S. Yuan, S. Jungsuttiwong, J. Photochem. Photobiol. A Chem. 322, 16 (2016)
D. Pugliese, A. Lamberti, F. Bella, A. Sacco, S. Bianco, E. Tressoa, Org. Electron. 15, 3715 (2014)
A. Yella, H.W. Lee, H.N. Tsao, A.K. Chandiran, M.K. Nazeeruddin, E.W.G. Diau, C.Y. Yeh, S.M. Zakeeruddin, M. Grätzel, Science 334, 629 (2011)
B. Nagarajan, S. Kushwaha, R. Elumalai, S. Mandal, K. Ramanujam, D. Raghavachari, J. Mater. Chem. A 5, 10289 (2017)
S. Chaurasia, J.T. Lin, Chem. Rec. 16, 1311 (2016)
A. Mahmood, Sol. Energy 123, 127 (2016)
B. Phillip, E.M. Louis, P. Adithya, N.I. Hammer, J.H. Delcamp, Synth. Met. 222, 66 (2015)
W.I. Hung, Y.Y. Liao, T.H. Lee, Y.C. Ting, J.S. Ni, W.S. Kao, J.T. Lin, Chem. Commun. 1, 2152 (2015)
Y.H. Numata, S. Zhang, X. Yang, L. Han, Chem. Lett. 42, 1328 (2013)
Y. Xie, Y. Tang, W. Wu, Y. Wang, J. Liu, X. Li, H. Tian, W.H. Zhu, J. Am. Chem. Soc. 137, 14055 (2015)
W. Zhang, Y. Wu, H. Zhu, Q. Chai, J. Liu, H. Li, X. Song, W. Zhu, ACS Appl. Mater. Interfaces 7, 26802 (2015)
S.G. Chen, H.L. Jia, X.H. Ju, X. Fang, L. Wang, H. Meie, Org. Lett. 13, 1610 (2011)
X. Ren, S. Jiang, M. Cha, G. Zhou, Z.S. Wang, Chem. Mater. 24, 3493 (2012)
Y.S. Yang, H.D. Kim, J.H. Ryu, K.K. Kim, S.S. Park, K.S. Ahn, J.H. Kim, Synth. Met. 161, 850 (2011)
Y. Hong, J.Y. Liao, J. Fu, X. Zang, D.B. Kuang, L. Wang, H. Meier, C.Y. Su, Dyes Pigments 94, 481 (2012)
M. Norberto, C. Bianca, A. Alessandro, Eur. J. Org. Chem. 32, 7069 (2014)
D. El-Sherbiny, H. Cheema, F. El-Essawy, A. Abdel-Megied, A. El-Shafei, Dyes Pigments 115, 81 (2015)
X.F. Zang, T.L. Zhang, Z.S. Huang, Z. Iqbal, D.B. Kuang, L. Wang, H. Meier, Dyes Pigments 104, 89 (2014)
Y.P. Hong, Z. Iqbal, X.L. Yin, D. Cao, Tetrahedron 70, 6296 (2014)
L. Agostina, D. Luisa, A. Giuseppina, Dyes Pigments 130, 79 (2016)
P. Bomben, K. Theriault, C. Berlinguette, Eur. J. Inorg. Chem. 2011, 1806 (2011)
J. Bisquert, Phys. Chem. Chem. Phys. 5, 5360 (2003)
J. Tang, J. Hua, W. Wu, J. Li, Z. Jin, Y. Long, H. Tian, Energy Environ. Sci. 3, 1736 (2010)
K. Hara, M. Kurashige, Y. Dan-oh, C. Kasada, A. Shinpo, S. Suga, K. Sayama, H. Arakawa, New J. Chem. 27, 783 (2003)
K. Funabiki, H. Mase, Y. Saito, A. Otsuka, A. Hibino, N. Tanaka, H. Miura, Y. Himori, T. Yoshida, Y. Kubota, M. Matsu, Org. Lett. 14, 1246 (2012)
F. Francisco, B. Juan, G. Germà, G. Boschloo, A. Hagfeldt, Sol. Energy Mater. Sol. Cells 87, 117 (2005)
H. Cheema, A. Islam, R. Younts, B. Gautam, I. Bedja, R.K. Gupta, L. Han, K. Gundogdu, A. El-Shafei, Phys. Chem. Chem. Phys. 16, 27078 (2014)
Acknowledgements
We gratefully acknowledge the financial supported by the funding of National Natural Science Foundation of China (21406202), Hangzhou Agricultural Scientific Research Project (20160432B25, 20180432B35), Zhejiang Public Welfare Technology Research Program (LGN19C200014), College Students in Zhejiang Province Sciences and Technology Innovation Activities (No. 2017R452002), and China Scholarship Council (File No. 201708330572).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lyu, L., Tang, P., Tong, G. et al. Molecular engineering and synthesis of symmetric metal-free organic sensitizers with A-π-D-π-A architecture for DSSC applications: the effect of bridge unit. J IRAN CHEM SOC 16, 2441–2450 (2019). https://doi.org/10.1007/s13738-019-01713-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01713-3