Abstract
The current epidemic of obesity in western countries is being worsened by the lack of effective pharmacotherapies. The apparent success of a central nervous system-acting cannabinoid CB1 receptor antagonist-based treatment for obesity was hampered by the appearance of psychiatric side effects in certain patients. These adverse effects forced its withdrawal from the market. However, the discovery that the main beneficial metabolic effects of cannabinoid CB1 receptor antagonists were derived of its activity in peripheral tissues, including the adipose tissue, opened the possibility of rescuing this type of therapy. This goal might be achieved by differential medicinal chemistry approaches. The present review examines these options that include peripheral-restricted cannabinoid CB1 receptor antagonists, dual ligands and combinatorial therapies using sub-effective doses of CB1 receptor antagonists that might be devoid of side effects.
Similar content being viewed by others
Introduction
In the last few decades, the incidence of overweight and obesity has grown to epidemic proportions. Obesity is a complex metabolic disorder characterized by an imbalance in energy homeostasis, abnormal increase of adipose tissue, and dysregulation of hormones, cytokines and other important signaling systems. This multi-factorial disorder is associated with co-morbidities such as cardiovascular risk, hypertension, sleep apnea, diabetes mellitus, hepatic steatosis and certain types of cancer among others. Its impact on national health systems has led to substantial research efforts towards the discovery of novel anti-obesity therapies.
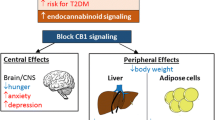
Among the new targets for pharmaceutical development of anti-obesity drugs, the endogenous cannabinoid system (ECS) remains a focus of attention. This signaling system is widely distributed in the central nervous system and peripheral tissues, and is involved in physiological actions related to food intake and energy homeostasis, predominantly via the cannabinoid type 1 receptor (CB1). Animal studies and clinical trials have shown that blockade of CB1 receptor induces weight loss, improves cardiometabolic risk factors and insulin resistance, and causes metabolic benefits. Therefore, all these data have emphasized the potential of CB1 receptor blockade as a therapeutic strategy in obesity.
Following this rationale, several cannabinoid receptors antagonists have been developed and some of them have reached clinical trials. One of them, rimonabant, eventually reached the market after approval by the European Medicines Agency. Nevertheless, the central actions of the prototypic CB1 receptor antagonist rimonabant have also been associated with the appearance, in clinical trials, of adverse psychiatric side effects, such as anxiety and depression, in patients treated for obesity. These adverse effects have motivated the withdrawal of rimonabant from the market. However, the question remains whether peripheral blockade of CB1 receptors is still an acceptable mechanism/strategy for the treatment of obesity. The aim of this review is twofold: to summarize the effects of CB1 receptor blockade in energy balance and to discuss the development of new approaches for obesity as effective therapies with reduced side effects.
The Endogenous Cannabinoid System
The ECS is a physiological signaling system which comprises cannabinoid receptors, endogenous ligands and enzymes responsible for the synthesis, transport and inactivation of these ligands [1•]. The best characterized endogenous cannabinoids are N-arachidonoylethanolamine (also known as anandamide, AEA) and 2-arachidonoylglycerol (2-AG), both derived from arachidonic acid conjugated with ethanolamine or glycerol, respectively [2–4]. Although AEA and 2-AG were originally identified as synaptic neuromodulators in neuronal systems, endocannabinoids are implicated in the regulation of several physiological processes in other non-nervous tissues. Therefore, endocannabinoids are present in brain, plasma, and peripheral tissues exerting agonist activity at cannabinoid receptors.
Two major types of cannabinoid receptors have been characterized and cloned: CB1 and CB2, both of which belong to the super-family of G protein-coupled receptors. While CB1 receptors are highly expressed in brain and are also found in peripheral tissues (e.g. muscle, gastrointestinal tract, pancreas, liver and adipose tissue) [5–8], CB2 receptors are mainly located in immune cells (T cells, B cells and monocytes) although there is evidence for their expression in both neurons and glial cells in the brain [9, 10]. In addition to classical cannabinoid receptors, other targets have been found to be modulated by endocannabinoids including the transient receptor potential vanilloid type 1 channel and orphan G protein-coupled receptors, such as GPR55 [11•].
Endocannabinoids are not stored in cellular vesicles, but are produced on demand, and rapidly degraded by intracellular enzymes; thus, the importance of the enzymes involved in their synthesis and degradation. The major route for AEA production is from phospholipid precursors through the action of N-acylphosphatidylethanolamine-selective phospholipase D (NAPE-PLD) [12], while 2-AG derives primarily from the hydrolytic metabolism of 1,2-diacylglycerol (DAG) mediated by two sn-1-selective DAG lipases, DAGLα and DAGLβ [13]. Inactivation of the endocannabinoid signaling is mediated by cellular reuptake and subsequent intracellular hydrolysis. Fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) have been identified as enzymes primarily responsible for the degradation of AEA and 2-AG, respectively [14, 15]. Additional metabolic pathways have been described using specific molecular inhibitors and genetic models lacking particular enzymes [16].
The ECS in Obesity
The widespread presence of the ECS indicates its physiological relevance in the regulation of a variety of biological processes (e.g. modulation of neural development, immune function, synaptic plasticity and learning, emotional state, pain…). In recent years, there has been substantial interest in investigating the role of endocannabinoid signaling in the regulation of metabolism and energy homeostasis, mainly due to the abundance of CB1 receptor expression in brain regions and peripheral tissues involved in the control of feeding behavior and energy balance. CB1 receptors, as well as endocannabinoid producing machinery have been described in the gut [17], liver [18], muscle [19•], endocrine pancreas [20] and white adipose tissue [21, 22]. In this regard, it is well known that activation of CB1 receptors promotes appetite and weight gain, while its blockade induces anorexia and weight loss with metabolic effects in mammals [23•].
Obesity is characterized by leptin and insulin resistance, and appears to be associated with a dysregulated and hyperactive ECS in rodents and humans. There is an increase in circulating endocannabinoid levels and altered expression of the components of this system in cerebral areas such as hypothalamus, hindbrain and limbic areas, but also in peripheral organs including adipose tissue, liver and pancreas [24].
In fact, diet-induced obese mice display an up-regulation of the components of the ECS in the hypothalamus and in other extrahypothalamic areas, such as the hippocampus and the extended amygdala, brain areas involved in the emotional aspects of eating [25, 26, 27•]. Similarly, high levels of endocannabinoids have been found in the hypothalamus of rodents, with an altered leptin signaling, such as db/db and ob/ob mice or Zucker fa/fa rats, which can be considered genetic models of obesity. This may be due to the fact that the biosynthesis of hypothalamic endocannabinoids is under the negative control of functional leptin [28].
In peripheral tissues and plasma, elevated endocannabinoid levels have also been found in obese compared to lean subjects. Recent clinical studies have shown that obesity is associated with elevated levels of 2-AG in visceral fat, a tissue that plays a main role in the development of metabolic syndrome, showing a positive correlation between visceral fat accumulation and circulating level of 2-AG. Interestingly, patients with type-2 diabetes have increased endocannabinoid levels in plasma [29, 30]. However, these elevations in endocannabinoids have also been reported in other feeding disorders such as anorexia nervosa and binge eating disorders. In all these cases, the elevated levels may be secondary to low levels of circulating leptin [31]. A recent study in healthy normal weight subjects has also demonstrated gender differences for endocannabinoids in plasma. Indeed, circulating levels of 2-AG are higher in males compared with females, as well as the existence of a strong correlation between 2-AG and triglyceride levels. In females, by contrast, these authors have observed an association between circulating AEA and adiposity and metabolic parameters [32•].
The increase in endocannabinoids may be caused by alterations in other enzymes related to the ECS. For instance, increased endocannabinoid levels may be due to the inhibition of the endocannabinoid-degrading enzyme FAAH, since a down-regulation of its gene expression and a decrease of its activity in adipose tissue and liver has been reported [18, 33]. Another recent clinical study has suggested that the dysregulation of ECS may result from a genetic predisposition. In this study, a significant association between a missense polymorphism in FAAH that leads to decreased enzymatic activity and overweight/obesity has been found [34]. Insulin might participate in such enzymatic regulation, since it has been reported that insulin decreases endocannabinoid levels and stimulates FAAH expression in adipose tissue of non-obese subjects, and therefore elevated endocannabinoid levels in obesity may also be due to insulin resistance [35].
In addition, the expression of CB1 receptors is also altered in obesity. In fact, increased concentrations of endocannabinoids may overstimulate CB1 receptors in a pathophysiological manner contributing to obesity [24, 29, 33, 36, 37]. However, the dysregulation of the CB1 receptor observed in peripheral organs is diverse throughout different animal studies and clinical trials with both elevations and reductions of its gene expression. Obese rodents display an increased CB1 gene expression in adipose tissue, liver and skeletal muscle [18, 38, 39] and such increased expression has also been observed in adipose tissue in human obesity [35, 40]. However, other studies reported no changes in CB1 gene expression in visceral and subcutaneous adipose tissue, or even reduced CB1 expression with obesity [33, 41]. This alteration of CB1 receptors has also been detected in the brain and, for instance, Zucker obese rats display a higher CB1 receptor binding than their lean counterparts in limbic regions, including reward-related brain areas that may contribute to hyperphagia [42].
Classical studies using cannabinoid CB1 receptor blockers show a more effective effect in obese rodents than in lean controls [38, 43, 44, 45•]. This increased sensitivity of obese animals can be explained by a higher sensitivity to the effects of CB1 antagonists due to the dysregulation and overactivation of the ECS. Another alternative explanation is related to the inverse agonism activity exhibited by these CB1 receptor blockers. However, CB1 receptor blockers identified as neutral CB1 antagonists have been reported to produce comparable metabolic benefits [46•].
Accordingly, pharmacological blockade of CB1 signaling represents an interesting approach for the development of new therapies against obesity. Consequently, several CB1 receptor antagonists and inverse agonists have been developed as anti-obesity drugs in recent years.
Central Versus Peripheral CB1 Receptor Blockade in Obesity: The Pros and Cons
Although early studies were focused on the ECS located in central nervous system circuits which regulate appetite and food intake, a growing body of evidence indicates that these functions are modulated through a combination of both central and peripheral mechanisms. CB1 receptor activation results in increased appetite [47, 48] and, although these receptors are highly expressed in cerebral areas involved in the regulation of motivated behaviors, they are also found in peripheral tissues with important functions related to the maintenance of energy balance. Thus, genetically modified mice lacking CB1 receptors eat less than wild-type littermates, even under fasting conditions. They show decreased body weight and reduced fat content compared to their controls, and are resistant to high-fat diet obesity [21, 28, 49]. Moreover, a continuous stimulation of CB1 receptors due to elevated levels of endocannabinoids leads to weight gain, enhancement of adiposity and a gradual worsening of cardiometabolic risk, and such a scenario is exactly observed in obesity with a dysregulated ECS.
Consequently, several cannabinoid CB1 receptor antagonists have been developed as anti-obesity drugs. Synthetic and plant-derived cannabinoid CB1 receptor blockers have been reported to suppress food intake, whereas chronic treatment leads to weight loss and improved metabolic profile in rodents including both genetic and dietary models of obesity [43, 44, 50, 51]. CB1 blockers have become a useful tool for the pharmacological characterization and for the elucidation of the molecular mechanism of action of the endocannabinoid signaling pathway.
The first selective CB1 receptor blocker, rimonabant (also referred to as SR141716A), is a diaryl-pyrazole derivative which has led to the development of several series of CB1 receptor antagonists/inverse agonists based on its structure and pharmacological properties (e.g. AM251 and surinabant) [52]. In addition, other non-diaryl-pyrazole derivatives have been evaluated in feeding behavior (e.g. taranabant) [53].
Animal studies and human trials, including obese subjects, have proven the efficacy of rimonabant in preventing cannabinoid effects as well as its efficacy as anorectic drug. Studies in animal models of obesity have evidenced that treatment with rimonabant results in body weight loss resulting attributable to a reduction in food intake, but also to an increase in whole-body energy expenditure with the involvement of several peripheral organs. Blockade of central and peripheral CB1 receptors by rimonabant has been shown to induce important weight loss and improvement in several metabolic parameters correcting hyperinsulinemia, lowering non-esterified ‘free’ fatty acid levels and reversing insulin resistance, all of which counteract the adverse effects of an overstimulated ECS [43, 44].
Central CB1 Receptor Blockade
The central blockade of cannabinoid receptors results in a significant inhibition of food intake in obese rats mainly through the CB1 receptor site in the hypothalamus, including ventromedial nucleus and lateral hypothalamus, and other interconnected cerebral areas [54]. Although the expression of CB1 receptor is not too high in the hypothalamus, it is more efficient than in other areas [55, 56].
Interestingly, systemic but not intracerebroventricular administration of CB1 antagonists blocks the food intake in food-deprived animals, suggesting the existence of a peripheral mechanism in the modulation of feeding [57]. However, local administration in determined brain areas blocks these actions. Indeed, conditional mutant mice characterized by a CB1 deletion in glutamatergic forebrain-projecting neurons in the hypothalamus and in the nucleus of the solitary tract are resistant to diet-induced obesity. Moreover, the treatment with rimonabant has no effect on body weight and food intake in these mutant mice [58•]. In this regard, a tolerance to suppressing-appetite effects has been observed in preclinical studies after chronic CB1 blockade, but not to the effect on body weight. This differential activity suggests certain independent mechanisms between food intake and body weight, which may be explained through opposite actions of the ECS in glutamatergic and GABAergic neurotransmission related to food intake [46•, 59•]. Because there is a bimodal action of CB1 receptor depending on its localization on GABA versus glutamatergic receptors, we cannot attribute feeding reduction to central effects exclusively.
A growing body of evidence suggests an interaction between the ECS and other orexigenic signals at the hypothalamic level, such as ghrelin and orexin systems. Thus, the ECS may be able to potentiate the stimulation of appetite process induced by these signaling systems. By contrast, both the genetic lack and pharmacological blockade of CB1 receptors eliminate these orexigenic effects [60–63]. Other polypeptide hormones in the hypothalamus are affected by endocannabinoid components; in fact, CB1 receptor knockout mice display altered levels of corticotropin-releasing hormone and cocaine- and amphetamine-related transcript [21]. In addition, another study has reported that treatment with rimonabant is also associated with an altered leptin signaling in the hypothalamus [64].
It is known that the hypothalamus is interconnected with the neuronal pathways of the reward system and that cannabinoids increase the intake of palatable food [48, 65, 66], implying a role in the modulation of brain reward mechanisms. The ECS has been reported in limbic areas, such as nucleus accumbens, ventral tegmental area, amygdala or prefrontal cortex suggesting an interaction with other signaling systems of neurotransmitters involved in feeding behavior, such as excitatory glutamate and inhibitory GABA transmissions and monoaminergic systems, mainly dopamine and serotonin [59•]. Although not fully explored, it is therefore reasonable to assume that such an influence results from interactions between the cannabinoid, opioid and dopamine systems in the regulation of palatable food consumption and reward [67–69]. In fact, rimonabant and other CB1 antagonists can cause comparable dose-dependent reductions in the consumption of both regular laboratory chow and palatable diets [70, 71]. However, CB1 receptor knockout mice do not display phenotypic differences in sucrose or food consumption [72, 73].
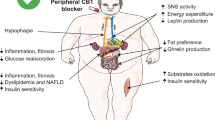
Peripheral CB1 Receptor Blockade
Despite the central mechanisms for the regulation of food intake by the ECS, there is evidence for the existence of peripheral mechanisms. Gomez and colleagues have described that rimonabant is able to reduce food intake after peripheral but not central administration in food-deprived rats, and sensory deafferentation by capsaicin prevents these peripheral actions on feeding [57]. These results suggest a main role of CB1 receptors at peripheral sensory nerve terminals, although central mechanisms are necessary to exert its actions. Consistent with this study, rimonabant has been reported to activate c-fos expression in brainstem receiving vagal inputs after systemic administration in rats [74•]. Therefore, the presence of cannabinoid receptors in peripheral organs has to perform additional short-term and long-term roles in appetite and metabolic changes/adaptations. In fact, the reduction of body weight (gain) and metabolic benefits by chronic treatment with CB1 blockers is more probable to be explained by peripheral than by central effects, at least, in rodent models of obesity [49].
Gastrointestinal Tract
It has been demonstrated that the level of endocannabinoids in the intestinal tract is short-term modulated by feeding status, with elevated levels following food deprivation and decreases during re-feeding in rats [57]. In addition, diet composition also modifies intestinal endocannabinoid levels and a fat-rich meal increases 2-AG and AEA levels [75]. By contrast, intestinal CB1 receptor blockade by rimonabant reduces fat intake in sham fed rats (experimental procedure by which animals receive the diet by a chronically implanted gastric cannula) [76•]. These results suggest a role of the ECS in the rewarding properties of fat-rich meals, driving to fat intake from the gastrointestinal tract. Besides, CB1 receptors present on enteric nerves throughout the wall of the gut are involved in the regulation of gastrointestinal motility in normal conditions, but such motility and secretion in the small intestine and the colon may be affected under pathophysiological conditions. Interestingly, CB1 inverse agonists and neutral antagonists have different effects on intestinal motility in mice, with a reduced gut transit observed only after rimonabant treatment. Dysregulated and overactivated ECS may be affecting the regular intestinal transit, which is related to eating disorders and gut side effects [77].
Liver
Initially, the presence of CB1 receptors in the mouse liver was confirmed indicating that hepatocytes could be a peripheral molecular target of the ECS [18]. Indeed, the activation of CB1 receptor increases the expression of lipogenic genes in the liver, which is the main source of de novo fatty acid synthesis in the body, and a pretreatment with CB1 receptor antagonists prevents this effect. Moreover, the basal rates of fatty acid synthesis and triglyceride storage are markedly increased in mice fed a high-fat diet, but not in CB1 knockout mice, which are resistant to the steatosis effect [18]. The pretreatment of these mice with a CB1 receptor antagonist reduces the rate of hepatic fatty acid synthesis, and, therefore, these data suggest the involvement of cannabinoid activation in liver steatosis associated with obesity [46•].
Skeletal Muscle
Growing evidence indicates that the ECS is involved in the regulation of glucose uptake and fatty acid oxidation pathways in skeletal muscle. CB1 receptors are found in human and rodent skeletal muscle and their continuous activation by high levels of endocannabinoids is associated with insulin resistance and with a decrease in glucose uptake by skeletal muscle cells [22, 78]. By contrast, several studies have reported a direct effect of CB1 blockade on energy expenditure and oxidative metabolism in skeletal muscle. Cavuoto and colleagues have shown that treatment with rimonabant affects the expression of genes involved in oxidative metabolism [79]. Therefore, CB1 receptor antagonists increase the glucose uptake in isolated soleus muscle of genetically obese mice and in cultured L6 skeletal muscle cells through the phosphatidylinositol-3-kinase pathway [39, 80]. Recently, it has been described an altered endocannabinoid signaling in muscle, as a result of a high-fat diet, and how the blockade of CB1 receptors could work towards restoration of the metabolic adaption imposed by diet [19•].
Adipose Tissue
While the effects of CB1 receptor blockers on food intake are transient, the body weight loss or gain inhibition is sustained over time. For this reason, the additional peripheral metabolic mechanisms have been proposed for the anti-obesity effects of this antagonism mainly in adipose tissue. CB1 receptors were initially found in epididymal fat pads and subsequently in other adipose tissues, and their activation in primary adipocytes from mice induced lipogenesis [21]. Therefore, the blockade of CB1 receptors in adipocyte is associated with an enhancement of lipid mobilization and a reduction of lipid storage, contributing to sustained weight loss [54]. Interestingly, in vivo studies and in adipocyte cultures treated with rimonabant result in an increase of adiponectin release, an adipokine involved in body weight regulation and homeostasis which is secreted by adipose tissue with plasma levels negatively correlated with obesity [81]. Another study using obese Zucker rats has also shown that rimonabant stimulates adiponectin expression [38]. Accordingly, in vivo studies with obese mice have demonstrated an improvement of the metabolic syndrome associated to obesity after systemic treatment with rimonabant by reducing the level of insulin, leptin and free fatty acids [43]. These effects are mediated via CB1 receptors since rimonabant has no effect on adiposity and plasma insulin levels in CB1 receptor knockout mice [49].
Endocrine Pancreas
It is now established the existence of a functional ECS in the endocrine pancreas, suggesting it as a potential site for endocannabinoid regulation of glucose homeostasis. Previous studies have described the presence of both CB1 and CB2 receptors in rodent pancreatic β- and non-β-cells [37, 82, 83]. Similarly, human islets of Langerhans also express cannabinoid receptors as well as the machinery involved in synthesis and degradation of 2-AG [20]. Several lines of evidence suggest that the ECS plays a role in the regulation of insulin secretion. In fact, in vitro studies have revealed that the activation of CB1 receptors stimulates insulin and glucagon secretion [20, 84]. By contrast, studies using islets from obese rats have demonstrated that the blockade of CB1 receptor by rimonabant has a direct effect on islets, reducing the basal insulin hypersecretion due to obesity [85].
Mitochondrial Cannabinoid CB1 Receptor
Recently, several studies have demonstrated a role of CB1 receptors in mitochondrial biogenesis, suggesting that these receptors may be a switch for mitochondrial activity that may be the target of future therapies.
CB1 receptors have been found in the mitochondria of brain neurons, controlling cellular respiration and energy production [86•]. While activation of these receptors decreases respiration through a cAMP receptor mechanism contributing to the control of neurotransmitter release, their blockade may have the opposite effect.
They are also found in brown and white adipose tissues or muscle. It is known that CB1 stimulation down-regulates mitochondrial biogenesis and function in adipose tissue, liver and skeletal muscle [87•]. In fact, diabetic/obese subjects show an impaired mitochondrial biogenesis in metabolically active tissues, likely due to the ECS overactivity [88]. By contrast, in vitro studies have demonstrated that rimonabant treatment promotes mitochondrial biogenesis in adipocytes by inducing the expression of the endothelial nitric oxide synthase (eNOS) [89]. Similarly, CB1 knockout mice display an increased mitochondrial biogenesis and eNOS expression compared to their wild-type littermates. Therefore, rimonabant is able to restore the down-regulation of mitochondrial biogenesis observed in adipocytes of mice fed a high-fat diet to normal levels, preventing the increase of body weight and adiposity [89]. Interestingly, a recent study with immortalized murine adipocytes has reported that CB1 inhibition directly promotes transdifferentiation of white adipocytes into a mitochondrial-rich brown fat phenotype, and therefore these actions on thermogenesis and insulin sensitivity may contribute to body weight loss and improve glucose homeostasis [90•].
Rimonabant in Clinical Trials
The effects of rimonabant have been extensively explored in humans through numerous clinical studies. In particular, overweight and obese patients have been included in the different Rimonabant in Obesity programs (RIO-Europe, RIO-Lipids, RIO-North America, RIO-Diabetes and RIO-Asia), displaying a significant body weight loss, waist circumference reduction and an improvement of cardiovascular risk factors at the end of these clinical trials with no differences between Caucasian and Asian ethnic groups [91–95]. Rimonabant reached the market after successful trials revealing both metabolic benefits and body weight reduction in overweight and obese subjects [91, 92]. However, chronic treatment with rimonabant increases the appearance of psychiatric adverse events, such as depressive mood disorders and anxiety, increasing the risk of suicide [96, 97]. Patients given rimonabant also show other adverse reactions, including nausea and dizziness. Similarly, previous studies in animals had reported that acute administration of rimonabant induces anxiety-like responses [98]. Due to the appearance of these adverse effects, rimonabant has been withdrawn from the market.
Is Still a Cannabinoid Receptor Antagonist a Response for Obesity?
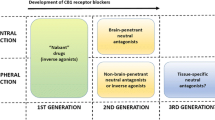
From the above discussed scientific evidence, it is clear that peripheral blockade of CB1 receptors may induce metabolic advantages for complicated obesity. However, a major goal is to avoid the unwanted central effects. The main critic to previous studies was the lack of control of the type of patients receiving treatment. The existence of previous affective disorders or the appearance of rimonabant-induced one was not controlled sufficiently. Now, after rimonabant withdrawal, non-centrally acting CB1 blockers would reach the clinic. Thus, the only option may be to exploit the peripheral blockade and this can be achieved through the following options: peripheral restricted antagonists that do not cross blood-brain barrier and dual ligands or combinatorial therapies that exploit synergies between CB1 antagonists/additional targets by enhancing thermogenesis and blocking lipid mobilization (Fig. 1).
Peripherally Restricted CB1 Receptor Antagonists
Since the psychiatric adverse effects observed in patients treated with rimonabant are derived from central CB1 receptor blockade, several new CB1 blockers with limited brain penetration are being synthesized and characterized to prevent adverse effects observed with rimonabant while maintaining anti-obesity properties.
Jagerovic and colleagues synthesized and characterized LH-21, a triazole derivative that acts as a neutral CB1 receptor antagonist [99], although other authors have described it as a weak CB1 receptor inverse agonist [100]. LH-21 displays a different pharmacological profile in comparison with rimonabant [101, 102]. Acute administration of this compound reduces food intake in fasted rats [101]. Similarly, subchronic treatment causes hypophagia and reduces body weight gain in obese rats, but with no metabolic effects [102, 103•]. LH-21 is more efficacious as anti-obesity drug in obese rats exposed to enriched-fat diet than in control rats, likely due to the dysregulation and overactivation of the ECS associated to obesity [103•]. Contrary to the metabolic benefits of rimonabant and AM251, obese rats treated with LH-21 do not display an improvement of the obesity-associated hepatic steatosis [103•]. The poor penetration of LH-21 into the central nervous system indicates that its anorectic effects are mainly mediated by peripheral CB1 receptors located in gut sensory terminals that control satiety [57], and also with cannabinoid receptors located in metabolically relevant tissues [21, 104]. Additionally, very high doses of LH-21 have been shown to have effects in CB1 receptor knockout mice, suggesting alternative targets for this compound [100]. A recent study has indicated that the anti-obesity properties of LH-21 may be mediated through modulation of the lipogenic pathways in adipose tissue [103•]. In adipocytes, LH-21 treatment also affects the gene expression of leptin, reducing its transcription and improving the leptin resistance associated to obesity [103•].
Another interesting compound is AM6545, a structurally modified analog of rimonabant, which acts as a neutral CB1 receptor antagonist with relatively poor penetrability into the brain [104]. This drug reduces food intake in animals fed high-fat and high-carbohydrate diets, but it is less effective in reducing regular chow intake [105]. Due to its low brain penetration, AM6545 does not affect behavioral responses mediated by CB1 receptors in the brain, such as catalepsy, hypomotility, hypothermia and anxiety-like behaviors [46•]. AM6545 reduces body weight gain and the adiposity index, improving the metabolic profile of obese mice fed a high-fat diet in a food intake-independent manner [104]. In fact, this drug has been reported to decrease lipogenic and increase lipolytic gene expression in both liver and adipose tissue. Moreover, AM6545 treatment reverses the hepatic steatosis induced by high-fat diet, reducing liver triglycerides and hepatocellular damage. By contrast, AM6545 has no effect on body weight and adiposity in ob/ob mice, likely due to leptin signaling deficiency [104]. Finally, AM6545 is also able to reduce food intake and body weight gain in CB1 receptor knockout mice, indicating the existence of other alternative pathways [106•].
URB447 is a pyrrole-derived compound with CB1 antagonist/ CB2 agonist properties and with reduced brain penetration. This drug decreases food intake and body weight gain in mice [107]. Recently, it has been reported that systemic administration of URB447 reduces fat intake in sham fed rats, supporting the anti-obesity properties of cannabinoid signaling blockade in the gut [76•].
In the last years, other compounds with reduced brain penetration have been described. For instance, JD-2114 and JD-5006 are non-brain penetrant CB1 antagonists that reduce body weight gain and improve metabolic parameters in obese mice maintained on a high-fat diet [108]. Son and colleagues have characterized a new rimonabant derivative acting peripherally and showing anti-obesity properties in diet-induced obese mice [109]. Recently, Receveur and colleagues have described another CB1 receptor antagonist with low brain penetration that reduces body weight gain in obese mice, although some actions of this compound at the central level should not be ruled out [110].
In summary, all these compounds with poor permeability into the central nervous system are able to induce weight loss without interfering with the neurobehavioral control of appetite, suggesting the importance of peripheral CB1 receptor blockade as anti-obesity target.
Efficacy of Combined Therapy for Obesity
The development of combined therapies is a new alternative in the field of obesity research. These types of therapy consist of the simultaneous use of at least two drugs targeting different systems involved in the regulation of feeding behavior and energy balance. One of the major benefits is that the co-administration of drugs may result in an additive or synergistic effect. Moreover, since combined therapies use low doses (sometimes sub-effective doses) of each compound, it is possible to minimize or avoid the adverse effects [111]. Several studies have proved the anti-obesity effects of low doses of CB1 receptor blockers when they are co-administered with different anorexigenic drugs as well as the blockade of orexigenic actions induced by other compounds.
Ghrelin is an appetite-stimulating peptide produced by the brain and gastrointestinal tract, mainly in the stomach [112]. The hyperphagic effects of this peptide are primarily through activation of the paraventricular and arcuate nuclei in the hypothalamus, both of which are involved in the control of appetite and energy balance [113]. Growing evidence suggests a functional interaction between brain ghrelin and ECS in appetite regulation. Thus, it has been demonstrated that intranuclear infusion of ghrelin into the paraventricular nucleus increases the feeding response in rats, an effect that is reversed by a non-effective, systemic dose of rimonabant [61, 62]. Pharmacological blockade of CB1 receptors also prevents the increase of 2-AG levels induced by ghrelin in the hypothalamus of mice [63]. Accordingly, no effects of ghrelin are observed in CB1 knockout mice [63].
The endogenous opioid system plays an important role in the control of food intake and so, the hyperphagic effects induced by opioids are well known [114]. Growing evidence suggests a cross-talk between the ECS and the endogenous opioid system in the regulation of appetite. Systemic and intra-hypothalamic injections of morphine promote appetite and this effect is abolished by peripheral administration of rimonabant [115]. A synergistic effect following a simultaneous blockade of cannabinoid and opioid receptors has also been described. Co-administration of sub-effective doses of rimonabant and the opioid antagonist naloxone decreases food intake in rats, enhancing the effects of each compound when they are given alone [116, 117]. Similar synergistic effects on food intake have been observed in mice given a combination of AM251 and the opioid antagonist nalmefene [118].
Orexin A (also called hypocretin 1) is a neuropeptide primarily involved in the stimulation of feeding. Direct infusions of orexin A into the lateral hypothalamus increase food intake in a dose-dependent manner [119]. Orexinergic neurons can integrate both central and peripheral signals regarding feeding and energy balance, interacting with other neurotransmission systems, including leptin, neuropeptide Y and ECS [120, 121]. Regarding this possible interaction between orexin and endocannabinoid systems, a co-expression of CB1 receptors and orexin receptor 1 in several brain regions, including the lateral hypothalamus has been described. [122], suggesting a cross-talk between both receptors [60]. In this regard, Crespo and colleagues have demonstrated that effective doses of rimonabant and sub-effective doses block the orexigenic actions of orexin A, indicating an interaction between both systems at hypothalamic levels [123].
The serotonergic system is also involved in the control of appetite and energy homeostasis. Systemic and intracerebral administrations of serotonin agonists reduce food intake and body weight [124, 125•]. A growing body of evidence suggests an interaction between serotonin and endocannabinoid systems in the regulation of appetite. Several brain area co-express serotonin and cannabinoid receptors [126]. Sibutramine, a serotonin- and noradrenalin-reuptake inhibitor, inhibits appetite, promotes weight loss and increases thermogenesis in brown adipose tissue [127, 128]. Besides, the administration of this drug is also associated to undesirable side-effects [129]. By contrast to previous studies that have reported an additive anti-obesity effect after co-administration of rimonabant and the serotonin-releasing compound D-fenfluramine [117] or synergistic interactions with serotonin agonists [130], the co-treatment of rimonabant and Sibutramine does not result in a significant anti-obesity effect [131•].
Oleoylethanolamide (OEA), a structurally AEA-related lipid with non-cannabinoid properties, is a mediator of satiety that exerts anorectic effects mainly through peripheral mechanisms [132]. Thus, a combinational therapy with OEA and rimonabant was suggested to enhance their respective beneficial actions as anti-obesity drugs. In this regard, previous studies have described a synergistic effect of both compounds to decrease appetite. A low, non-effective dose of rimonabant potentiates the inhibitory actions of OEA on feeding in food-deprived rats [57, 133]. In obese Zucker rats, a subchronic treatment with both drugs improves the separate effects of rimonabant and OEA, resulting in a marked decrease on feeding, body weight gain and serum lipid levels [133]. Additionally, this combinational therapy reduces the hepatic steatosis observed in obese rats, decreasing liver fat depots and improving liver function [133]. A similar effect has been observed with the combination of LH-21 and OEA [102].
Cholecystokinin (CCK) is another peripheral hormone that acts as a satiety factor, mainly through CCK1 receptors. The co-localization of these receptors with the CB1 receptors in peripheral nerve terminals of the gut, suggests an interaction between both receptors. Recently, an additive satiety induction has been reported in rats following the co-administration of rimonabant and a CCK1 agonist [74•].
Development of Novel Dual Ligands
Although the traditional strategy in medicinal chemistry of one disease /one target is still valid, the idea of a multi-target approach is gradually gaining interest especially in the case of complex diseases such as obesity as has already been mentioned. Some recent examples include combined administration of rimonabant and orlistat [134], and rimonabant and the melanin concentrating hormone antagonist SNAP-94847 [135•].
However, a different strategy is to design a compound with two pharmacophoric groups capable of interacting simultaneously with two different biological targets. These are the so-called designed multiple ligands which are being the subject of intense research in different therapeutic areas [136].
In this context, fatty acid amides of aryl pyrazoles, related to rimonabant and OEA, have been synthesized as dual CB1/ PPARα ligands. Although the compounds did not show significant cannabinoid properties certain derivatives were able to reduce food intake in rats, some through PPARα activation and others through an unknown mechanism [137]. More recently, rimonabant has been fused to fibrate resulting in compounds with nanomolar affinity for both cannabinoid and PPARα receptors, one of them being CB1 selective [138•].
Taking into account the complexity of obesity and eating disorders, DML´s can be considered an interesting approach although there is still considerable research to be done in this field.
Conclusions
The clinical experience with the cannabinoid CB1 receptor blocker rimonabant revealed the benefits of blocking cannabinoid receptors in complicated obesity. It also indicated that central effects on affective behaviors would be the limit for its therapeutic use. Targeting the peripheral cannabinoid receptors with antagonists that do not cross the blood-brain barrier might be an alternative for exploiting the benefits derived of reducing the impact of the overactive endocannabinoid system in obesity. Alternatives may derive of exploiting peripheral synergies such as those derived of simultaneous target of cannabinoid CB1 receptors and either PPARα receptors of peptide receptors such as CCK or ghrelin receptors. Synergism means greater efficacy at doses devoid of unwanted central effects, offering tissue specificity (depending on target expression and the combination of drugs selected). With these premises, a second opportunity for cannabinoid receptor antagonism might be set in place for complicated obesity.
References
Papers of particular interest, published recently, have been highlighted as:• Of importance
• Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588–631. The most recent consensus on the identification, description, nomenclature and properties of cannabinoid receptors and their endogenous ligands.
Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–9.
Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90.
Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215(1):89–97.
Batkai S, Jarai Z, Wagner JA, et al. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7(7):827–32.
Herkenham M, Lynn AB, Johnson MR, et al. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11(2):563–83.
Herkenham M, Lynn AB, Little MD, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87(5):1932–6.
Howlett AC, Bidaut-Russell M, Devane WA, et al. The cannabinoid receptor: biochemical, anatomical and behavioral characterization. Trends Neurosci. 1990;13(10):420–3.
Onaivi ES, Ishiguro H, Gong JP, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–36.
Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–5.
• Godlewski G, Offertaler L, Wagner JA, et al. Receptors for acylethanolamides-GPR55 and GPR119. Prostaglandins Other Lipid Mediat. 2009;89(3–4):105–11. Two new orphan receptors as targets of endogenous lipid transmitters related to the endogenous cannabinoid system. While Lysophosphatidyl inositol activates GPR55, oeloylethanolamide is proposed to be the endogenous ligand of GPR119. Both are relevant targets in obesity and type 2 diabetes.
Okamoto Y, Morishita J, Tsuboi K, et al. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279(7):5298–305.
Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388(6644):773–8.
Cravatt BF, Giang DK, Mayfield SP, et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384(6604):83–7.
Dinh TP, Carpenter D, Leslie FM, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99(16):10819–24.
Ueda N, Tsuboi K, Uyama T. Enzymological studies on the biosynthesis of N-acylethanolamines. Biochim Biophys Acta. 2010;1801(12):1274–85.
Marquez L, Suarez J, Iglesias M, et al. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS One. 2009;4(9):e6893.
Osei-Hyiaman D, DePetrillo M, Pacher P, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115(5):1298–305.
• Crespillo A, Suarez J, Bermudez-Silva FJ, et al. Expression of the cannabinoid system in muscle: effects of a high-fat diet and CB1 receptor blockade. Biochem J. 2011;433(1):175–85. The endogenous cannabinoid system is in the muscle. This paper describes its presence and the impact of caloric diets on its expression and fucntion.
Bermudez-Silva FJ, Suarez J, Baixeras E, et al. Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia. 2008;51(3):476–87.
Cota D, Marsicano G, Tschop M, et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest. 2003;112(3):423–31.
Pagotto U, Marsicano G, Cota D, et al. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev. 2006;27(1):73–100.
• Quarta C, Mazza R, Obici S, et al. Energy balance regulation by endocannabinoids at central and peripheral levels. Trends Mol Med. 2011;17(9):518–26. This review addresses the general role of the ECS in the regulation of energu expenditure, and discuss the contribution of central (CNS) and peripheral (adipose tissue, liver, pancreas) organs.
Engeli S. Dysregulation of the endocannabinoid system in obesity. J Neuroendocrinol. 2008;20 Suppl 1:110–5.
Di Marzo V. CB1 receptor antagonism: biological basis for emtabolic effects. Drug Discovery Today. 2008;13(23/24):1026–41.
South T, Huang XF. Temporal and site-specific brain alterations in CB1 receptor binding in high fat diet-induced obesity in C57Bl/6 mice. J Neuroendocrinol. 2008;20(11):1288–94.
• Massa F, Mancini G, Schmidt H, et al. Alterations in the hippocampal endocannabinoid system in diet-induced obese mice. J Neurosci. 2010;30(18):6273–81. This paper describes how high fat diet affects the endogenous cannabinoid system in the hippocampus. It clearly establish that nutritional aspects might regulate this system, so a net behavioural contribution can be expects as results of obesity-induced ECS alterations.
Di Marzo V, Goparaju SK, Wang L, et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410(6830):822–5.
Bluher M, Engeli S, Kloting N, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55(11):3053–60.
Matias I, Gonthier MP, Orlando P, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91(8):3171–80.
Monteleone P, Matias I, Martiadis V, et al. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology. 2005;30(6):1216–21.
• Fanelli F, Di Lallo VD, Belluomo I, et al. Estimation of reference intervals of five endocannabinoids and endocannabinoid related compounds in human plasma by two dimensional-LC/MS/MS. J Lipid Res. 2012;53(3):481–93. First general study on the fluctuations of plasma endocannabinoids in general population that also addresses the impact of obesity. This study also analyze the net effect of different biochemical procedures for estimating plasma concentrations of endocannabinoids.
Engeli S, Bohnke J, Feldpausch M, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54(10):2838–43.
Sipe JC, Waalen J, Gerber A, et al. Overweight and obesity associated with a missense polymorphism in fatty acid amide hydrolase (FAAH). Int J Obes (Lond). 2005;29(7):755–9.
Murdolo G, Kempf K, Hammarstedt A, et al. Insulin differentially modulates the peripheral endocannabinoid system in human subcutaneous abdominal adipose tissue from lean and obese individuals. J Endocrinol Invest. 2007;30(8):RC17–21.
Matias I, Petrosino S, Racioppi A, et al. Dysregulation of peripheral endocannabinoid levels in hyperglycemia and obesity: effect of high fat diets. Mol Cell Endocrinol. 2008;286(1-2 Suppl 1):S66–78.
Starowicz KM, Cristino L, Matias I, et al. Endocannabinoid dysregulation in the pancreas and adipose tissue of mice fed with a high-fat diet. Obesity (Silver Spring). 2008;16(3):553–65.
Bensaid M, Gary-Bobo M, Esclangon A, et al. The cannabinoid CB1 receptor antagonist SR141716 increases Acrp30 mRNA expression in adipose tissue of obese fa/fa rats and in cultured adipocyte cells. Mol Pharmacol. 2003;63(4):908–14.
Liu YL, Connoley IP, Wilson CA, et al. Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes (Lond). 2005;29(2):183–7.
Pagano C, Pilon C, Calcagno A, et al. The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab. 2007;92(12):4810–9.
Kempf K, Hector J, Strate T, et al. Immune-mediated activation of the endocannabinoid system in visceral adipose tissue in obesity. Horm Metab Res. 2007;39(8):596–600.
Thanos PK, Ramalhete RC, Michaelides M, et al. Leptin receptor deficiency is associated with upregulation of cannabinoid 1 receptors in limbic brain regions. Synapse. 2008;62(9):637–42.
Ravinet Trillou C, Arnone M, Delgorge C, et al. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R345–53.
Vickers SP, Webster LJ, Wyatt A, et al. Preferential effects of the cannabinoid CB1 receptor antagonist, SR 141716, on food intake and body weight gain of obese (fa/fa) compared to lean Zucker rats. Psychopharmacology (Berl). 2003;167(1):103–11.
• Judge MK, Zhang Y, Scarpace PJ. Responses to the cannabinoid receptor-1 antagonist, AM251, are more robust with age and with high-fat feeding. J Endocrinol. 2009;203(2):281–90. This paper addresses the obese-phenotype dependency of the pharmacological response to cannabinoid receptor antagonists.
• Kunos G, Tam J. The case for peripheral CB receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br J Pharmacol. 2011;163(7):1423–31. Analysis of the potential role of peripheral cannabinoid receptors in obesity and the impact of therapies aiming at this peripheral target.
Williams CM, Rogers PJ, Kirkham TC. Hyperphagia in pre-fed rats following oral delta9-THC. Physiol Behav. 1998;65(2):343–6.
Williams CM, Kirkham TC. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl). 1999;143(3):315–7.
Ravinet Trillou C, Delgorge C, Menet C, et al. CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord. 2004;28(4):640–8.
Thornton-Jones ZD, Kennett GA, Benwell KR, et al. The cannabinoid CB1 receptor inverse agonist, rimonabant, modifies body weight and adiponectin function in diet-induced obese rats as a consequence of reduced food intake. Pharmacol Biochem Behav. 2006;84(2):353–9.
Riedel G, Fadda P, McKillop-Smith S, et al. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br J Pharmacol. 2009;156(7):1154–66.
Rinaldi-Carmona M, Barth F, Heaulme M, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350(2–3):240–4.
Fong TM, Guan XM, Marsh DJ, et al. Antiobesity efficacy of a novel cannabinoid-1 receptor inverse agonist, N-[(1S,2S)-3-(4-chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-[[5-(t rifluoromethyl)pyridin-2-yl]oxy]propanamide (MK-0364), in rodents. J Pharmacol Exp Ther. 2007;321(3):1013–22.
Nogueiras R, Veyrat-Durebex C, Suchanek PM, et al. Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes. 2008;57(11):2977–91.
Tsou K, Brown S, Sanudo-Pena MC, et al. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411.
Breivogel CS, Sim LJ, Childers SR. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J Pharmacol Exp Ther. 1997;282(3):1632–42.
Gomez R, Navarro M, Ferrer B, et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22(21):9612–7.
• Quarta C, Bellocchio L, Mancini G, et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab. 2010;11(4):273–85. This important study clearly establish the role of cannabinoid CB1 receptor located in autonomic adrenergic neurons on the control of weight gain and energy expenditure.
• Bellocchio L, Lafenetre P, Cannich A, et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci. 2010;13(3):281–3. Identification of the opposite role of CB1 receptors located in either GABA or GLUTAMATE neurons on feeding. It sets the molecular basis for the biphasic effects of cannabinoids on feeding behaviour and other motivated behaviours.
Hilairet S, Bouaboula M, Carriere D, et al. Hypersensitization of the Orexin 1 receptor by the CB1 receptor: evidence for cross-talk blocked by the specific CB1 antagonist, SR141716. J Biol Chem. 2003;278(26):23731–7.
Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50(11):2540–7.
Tucci SA, Rogers EK, Korbonits M, et al. The cannabinoid CB1 receptor antagonist SR141716 blocks the orexigenic effects of intrahypothalamic ghrelin. Br J Pharmacol. 2004;143(5):520–3.
Kola B, Farkas I, Christ-Crain M, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One. 2008;3(3):e1797.
Lazzari P, Sanna A, Mastinu A, et al. Weight loss induced by rimonabant is associated with an altered leptin expression and hypothalamic leptin signaling in diet-induced obese mice. Behav Brain Res. 2011;217(2):432–8.
Koch JE. Delta(9)-THC stimulates food intake in Lewis rats: effects on chow, high-fat and sweet high-fat diets. Pharmacol Biochem Behav. 2001;68(3):539–43.
Koch JE, Matthews SM. Delta9-tetrahydrocannabinol stimulates palatable food intake in Lewis rats: effects of peripheral and central administration. Nutr Neurosci. 2001;4(3):179–87.
Cooper SJ. Endocannabinoids and food consumption: comparisons with benzodiazepine and opioid palatability-dependent appetite. Eur J Pharmacol. 2004;500(1–3):37–49.
Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81(2):263–84.
Cota D, Tschop MH, Horvath TL, et al. Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev. 2006;51(1):85–107.
McLaughlin PJ, Winston K, Swezey L, et al. The cannabinoid CB1 antagonists SR 141716A and AM 251 suppress food intake and food-reinforced behavior in a variety of tasks in rats. Behav Pharmacol. 2003;14(8):583–8.
Verty AN, McGregor IS, Mallet PE. Consumption of high carbohydrate, high fat, and normal chow is equally suppressed by a cannabinoid receptor antagonist in non-deprived rats. Neurosci Lett. 2004;354(3):217–20.
Hungund BL, Szakall I, Adam A, et al. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84(4):698–704.
Naassila M, Pierrefiche O, Ledent C, et al. Decreased alcohol self-administration and increased alcohol sensitivity and withdrawal in CB1 receptor knockout mice. Neuropharmacology. 2004;46(2):243–53.
• Orio L, Crespo I, Lopez-Moreno JA, et al. Additive effects of cannabinoid CB1 receptors blockade and cholecystokinin on feeding inhibition. Pharmacol Biochem Behav. 2011;98(2):220–6. This study show how the ECS opposes to the anorectic actions of CCK in peripheral nerve terminals. It demonstrates that CCK and a CB1 receptor antagonist can be additive in reducing feeding.
Artmann A, Petersen G, Hellgren LI, et al. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;1781(4):200–12.
• DiPatrizio NV, Astarita G, Schwartz G, et al. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci U S A. 2011;108(31):12904–8. The ECS not only controls appetite, but specific appetites such as that of fat. The study identifies the places throughout the whole gut where this inhibitory signal occurs.
Storr MA, Bashashati M, Hirota C, et al. Differential effects of CB(1) neutral antagonists and inverse agonists on gastrointestinal motility in mice. Neurogastroenterol Motil. 2010;22(7):787–96. e223.
Cavuoto P, McAinch AJ, Hatzinikolas G, et al. The expression of receptors for endocannabinoids in human and rodent skeletal muscle. Biochem Biophys Res Commun. 2007;364(1):105–10.
Cavuoto P, McAinch AJ, Hatzinikolas G, et al. Effects of cannabinoid receptors on skeletal muscle oxidative pathways. Mol Cell Endocrinol. 2007;267(1–2):63–9.
Esposito I, Proto MC, Gazzerro P, et al. The cannabinoid CB1 receptor antagonist rimonabant stimulates 2-deoxyglucose uptake in skeletal muscle cells by regulating the expression of phosphatidylinositol-3-kinase. Mol Pharmacol. 2008;74(6):1678–86.
Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83.
Juan-Pico P, Fuentes E, Bermudez-Silva FJ, et al. Cannabinoid receptors regulate Ca(2+) signals and insulin secretion in pancreatic beta-cell. Cell Calcium. 2006;39(2):155–62.
Bermudez-Silva FJ, Sanchez-Vera I, Suarez J, et al. Role of cannabinoid CB2 receptors in glucose homeostasis in rats. Eur J Pharmacol. 2007;565(1–3):207–11.
Vilches-Flores A, Delgado-Buenrostro NL, Navarrete-Vazquez G, et al. CB1 cannabinoid receptor expression is regulated by glucose and feeding in rat pancreatic islets. Regul Pept. 2010;163(1–3):81–7.
Getty-Kaushik L, Richard AM, Deeney JT, et al. The CB1 antagonist rimonabant decreases insulin hypersecretion in rat pancreatic islets. Obesity (Silver Spring). 2009;17(10):1856–60.
• Benard G, Massa F, Puente N, et al. Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat Neurosci. 2012;15(4):558–64. This study reveals a novel mechanism for the metabolic actions of endocannabinoids. The presence of CB1 receptors in the membrane of a relevante percentage of neuronal mitochdria allows the ECS to control respiration and neurotransmission.
• Tedesco L, Valerio A, Dossena M, et al. Cannabinoid receptor stimulation impairs mitochondrial biogenesis in mouse white adipose tissue, muscle, and liver: the role of eNOS, p38 MAPK, and AMPK pathways. Diabetes. 2010;59(11):2826–36. The activation of the ECS has a negative impact on mitochondrial development. This paper identifies the role of the ECS on mitochondriogenensis and explains the beneficial role of cannabinoid CB1 receptor antagonists on the number of active mitochondria.
Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31(3):364–95.
Tedesco L, Valerio A, Cervino C, et al. Cannabinoid type 1 receptor blockade promotes mitochondrial biogenesis through endothelial nitric oxide synthase expression in white adipocytes. Diabetes. 2008;57(8):2028–36.
• Perwitz N, Wenzel J, Wagner I, et al. Cannabinoid type 1 receptor blockade induces transdifferentiation towards a brown fat phenotype in white adipocytes. Diabetes Obes Metab. 2010;12(2):158–66. This paper sets the role for the peripheral CB1 receptor on brown to white adipocyte metaplasia. This mechanisms is sufficient to induce net weight loss.
Van Gaal LF, Rissanen AM, Scheen AJ, et al. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365(9468):1389–97.
Pi-Sunyer FX, Aronne LJ, Heshmati HM, et al. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA. 2006;295(7):761–75.
Despres JP, Golay A, Sjostrom L. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353(20):2121–34.
Pan C, Yoo HJ, Ho LT. Perspectives of CB1 Antagonist in Treatment of Obesity: Experience of RIO-Asia. J Obes. 2011;2011:957268.
Scheen AJ, Finer N, Hollander P, et al. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368(9548):1660–72.
Christensen R, Kristensen PK, Bartels EM, et al. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370(9600):1706–13.
Christopoulou FD, Kiortsis DN. An overview of the metabolic effects of rimonabant in randomized controlled trials: potential for other cannabinoid 1 receptor blockers in obesity. J Clin Pharm Ther. 2011;36(1):10–8.
Navarro M, Hernandez E, Munoz RM, et al. Acute administration of the CB1 cannabinoid receptor antagonist SR 141716A induces anxiety-like responses in the rat. Neuroreport. 1997;8(2):491–6.
Jagerovic N, Hernandez-Folgado L, Alkorta I, et al. Discovery of 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1 h-1,2,4-triazole, a novel in vivo cannabinoid antagonist containing a 1,2,4-triazole motif. J Med Chem. 2004;47(11):2939–42.
Chen RZ, Frassetto A, Lao JZ, et al. Pharmacological evaluation of LH-21, a newly discovered molecule that binds to cannabinoid CB1 receptor. Eur J Pharmacol. 2008;584(2–3):338–42.
Pavon FJ, Bilbao A, Hernandez-Folgado L, et al. Antiobesity effects of the novel in vivo neutral cannabinoid receptor antagonist 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-hexyl-1 H–1,2,4-triazole–LH 21. Neuropharmacology. 2006;51(2):358–66.
Pavon FJ, Serrano A, Perez-Valero V, et al. Central versus peripheral antagonism of cannabinoid CB1 receptor in obesity: effects of LH-21, a peripherally acting neutral cannabinoid receptor antagonist, in Zucker rats. J Neuroendocrinol. 2008;20 Suppl 1:116–23.
• Alonso M, Serrano A, Vida M, et al. Anti-obesity efficacy of LH-21, a cannabinoid CB(1) receptor antagonist with poor brain penetration, in diet-induced obese rats. Br J Pharmacol. 2012;165(7):2274–91. This study describes the effects of a cannabinoid CB1 receptor antagonist with poor brain penetration on diet-induced obese rats, identiying the adipose tissue as main target for its antiobesity actvity.
Tam J, Vemuri VK, Liu J, et al. Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest. 2010;120(8):2953–66.
Randall PA, Vemuri VK, Segovia KN, et al. The novel cannabinoid CB1 antagonist AM6545 suppresses food intake and food-reinforced behavior. Pharmacol Biochem Behav. 2010;97(1):179–84.
• Cluny NL, Vemuri VK, Chambers AP, et al. A novel peripherally restricted cannabinoid receptor antagonist, AM6545, reduces food intake and body weight, but does not cause malaise, in rodents. Br J Pharmacol. 2010;161(3):629–42. Another description of a molecule that blocks cannabinoid receptors but does not penetrate into the brain. This molecule reduces appetite and body weight gain without inducing malaise.
LoVerme J, Duranti A, Tontini A, et al. Synthesis and characterization of a peripherally restricted CB1 cannabinoid antagonist, URB447, that reduces feeding and body-weight gain in mice. Bioorg Med Chem Lett. 2009;19(3):639–43.
McElroy J, Sieracki K, Chorvat R. Non-brain-penetrant CB1 receptor antagonists as a novel treatment of obesity and related metabolic disorders. Obesity (Silver Spring). 2008;16(S47).
Son MH, Kim HD, Chae YN, et al. Peripherally acting CB1-receptor antagonist: the relative importance of central and peripheral CB1 receptors in adiposity control. Int J Obes (Lond). 2010;34(3):547–56.
Receveur JM, Murray A, Linget JM, et al. Conversion of 4-cyanomethyl-pyrazole-3-carboxamides into CB1 antagonists with lowered propensity to pass the blood-brain-barrier. Bioorg Med Chem Lett. 2010;20(2):453–7.
Greenway FL, Whitehouse MJ, Guttadauria M, et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring). 2009;17(1):30–9.
Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60.
Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–8.
Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25(4):697–725.
Verty AN, Singh ME, McGregor IS, et al. The cannabinoid receptor antagonist SR 141716 attenuates overfeeding induced by systemic or intracranial morphine. Psychopharmacology (Berl). 2003;168(3):314–23.
Kirkham TC, Williams CM. Synergistic efects of opioid and cannabinoid antagonists on food intake. Psychopharmacology (Berl). 2001;153(2):267–70.
Rowland NE, Mukherjee M, Robertson K. Effects of the cannabinoid receptor antagonist SR 141716, alone and in combination with dexfenfluramine or naloxone, on food intake in rats. Psychopharmacology (Berl). 2001;159(1):111–6.
Chen RZ, Huang RR, Shen CP, et al. Synergistic effects of cannabinoid inverse agonist AM251 and opioid antagonist nalmefene on food intake in mice. Brain Res. 2004;999(2):227–30.
Thorpe AJ, Mullett MA, Wang C, et al. Peptides that regulate food intake: regional, metabolic, and circadian specificity of lateral hypothalamic orexin A feeding stimulation. Am J Physiol Regul Integr Comp Physiol. 2003;284(6):R1409–17.
Haynes AC, Jackson B, Overend P, et al. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides. 1999;20(9):1099–105.
Horvath TL, Diano S, van den Pol AN. Synaptic interaction between hypocretin (orexin) and neuropeptide Y cells in the rodent and primate hypothalamus: a novel circuit implicated in metabolic and endocrine regulations. J Neurosci. 1999;19(3):1072–87.
Hervieu GJ, Cluderay JE, Harrison DC, et al. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103(3):777–97.
Crespo I, Gomez de Heras R, Rodriguez de Fonseca F, et al. Pretreatment with subeffective doses of Rimonabant attenuates orexigenic actions of orexin A-hypocretin 1. Neuropharmacology. 2008;54(1):219–25.
Simansky KJ. Serotonergic control of the organization of feeding and satiety. Behav Brain Res. 1996;73(1–2):37–42.
• Garfield AS, Heisler LK. Pharmacological targeting of the serotonergic system for the treatment of obesity. J Physiol. 2009;587(Pt 1):49–60. A review on the role of serotonin in obesity and the effects of serotonergic drugs. Lorcaserin, a 5HT2C receptor agonist is the first drug approved for obesity after the case of the CB1 receptor antagonist Rimonabant.
Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109(3):451–60.
Jackson HC, Needham AM, Hutchins LJ, et al. Comparison of the effects of sibutramine and other monoamine reuptake inhibitors on food intake in the rat. Br J Pharmacol. 1997;121(8):1758–62.
Casado A, Rodriguez VM, Portillo MP, et al. Sibutramine decreases body weight gain and increases energy expenditure in obese Zucker rats without changes in NPY and orexins. Nutr Neurosci. 2003;6(2):103–11.
Luque CA, Rey JA. The discovery and status of sibutramine as an anti-obesity drug. Eur J Pharmacol. 2002;440(2–3):119–28.
Ward SJ, Lefever TW, Jackson C, et al. Effects of a Cannabinoid1 receptor antagonist and Serotonin2C receptor agonist alone and in combination on motivation for palatable food: a dose-addition analysis study in mice. J Pharmacol Exp Ther. 2008;325(2):567–76.
• Tallett AJ, Blundell JE, Rodgers RJ. Effects of acute low-dose combined treatment with rimonabant and sibutramine on appetite and weight gain in rats. Pharmacol Biochem Behav. 2010;97(1):92–100. An example of combinatorial therapy in obesity: the combination of subeffective doses of a serotonin uptake inhibitor and a cannabinoid receptor antagonist.
Rodriguez De Fonseca F, Navarro M, Gomez R, et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414(6860):209–12.
Serrano A, Del Arco I, Javier Pavon F, et al. The cannabinoid CB1 receptor antagonist SR141716A (Rimonabant) enhances the metabolic benefits of long-term treatment with oleoylethanolamide in Zucker rats. Neuropharmacology. 2008;54(1):226–34.
Zaitone SA, Essawy S. Addition of a low dose of rimonabant to orlistat therapy decreases weight gain and reduces adiposity in dietary obese rats. Clin Exp Pharmacol Physiol. 2012;39(6):551–9.
• Verty AN, Lockie SH, Stefanidis A, et al. Anti-obesity effects of the combined administration of CB1 receptor antagonist rimonabant and melanin-concentrating hormone antagonist SNAP-94847 in diet-inudced obese mice. Int J Obes (Lond). 2012. Another example of a combinatorial therapy based on the association of a cannabinoid CB1 receptor antagonist and a melanin-concentrating hormone antagonist.
Costantino L, Barlocco D. Designed Multiple Ligands: Basic Research vs Clinical Outcomes. Curr Med Chem. 2012;19(20):3353–87.
Alvarado M, Goya P, Macias-Gonzalez M, et al. Antiobesity designed multiple ligands: Synthesis of pyrazole fatty acid amides and evaluation as hypophagic agents. Bioorg Med Chem. 2008;16(23):10098–105.
• Perez-Fernandez RF, N; Macias, M; Elguero, J, et al. Dicovery of potent dual PPAR alpha agonists / CB1 ligands. Med Chem Lett. 2011:793-7. This paper demonstrates that it is possible to create new chemical entities based in known cannabinoid CB1 receptor ligands that act as dual drugs: in this case, the second target is the PPARα receptor.
Disclosure
Conflicts of interest: A. Serrano: none; F.J. Pavon :none; J. Suarez: none; M. Romero-Cuevas: none; E. Baixeras; none; P. Goya: none; F. Rodríguez de Fonseca: has received research grants from VIVIA Biotech SL and Zeltia SA.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Serrano, A., Pavon, F.J., Suarez, J. et al. Obesity and the Endocannabinoid System: Is There Still a Future for CB1 Antagonists in Obesity?. Curr Obes Rep 1, 216–228 (2012). https://doi.org/10.1007/s13679-012-0031-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-012-0031-x