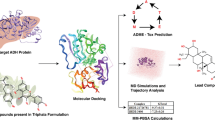
Abstract
11β-Hydroxysteroid dehydrogenase 1 (11β-HSD1) represents a promising drug target for metabolic syndrome, including obesity and type 2 diabetes. Our initial screen of a collection of natural products from Danshen led to the identification of tanshinones as the potent and selective 11β-HSD1 inhibitors. To improve the druggability and explore the structure–activity relationships (SARs), more than 40 derivatives have been designed and synthesized using tanshinone IIA and cryptotanshinone as the starting materials. More than 10 derivatives exhibited potent in vitro 11β-HSD1 inhibitory activity and good selectivity over 11β-HSD2 across human and mouse species. Based on the biological results, SARs were further discussed, which was also partially rationalized by a molecular docking model of 1 bound to the 11β-HSD1. Remarkably, compounds 1, 17 and 30 significantly inhibited 11β-HSD1 in 3T3-L1 adipocyte and in livers of ob/ob mice, which merits further investigations as anti-diabetic agents. This study not only provides a series of novel selective 11β-HSD1 inhibitors with promising therapeutic potentials in metabolic syndromes, but also expands the boundaries of the chemical and biological spaces of tanshinones.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metabolic syndrome is a prediabetic state, which features central obesity, glucose intolerance, insulin resistance, dyslipidemia, hypertension, and inflammatory or prothrombotic state [1]. Metabolic syndrome can progress to type 2 diabetes, which results in serious diabetes complications, including cardiovascular disease, diabetic retinopathy, renal failure, nerve damage and ischemic stroke [2]. It is postulated that aberrant glucocorticoid signaling may contribute to the metabolic syndrome [3]. Increased local concentration of glucocorticoids rather than systematically elevated glucocorticoid levels impairs insulin and leptin sensitivity, which ultimately results in metabolic disease, including Cushing’s syndrome, obesity and type 2 diabetes [4]. These findings suggest that control of intracellular glucocorticoid level (specifically intracellular concentration in the metabolically relevant tissues) is a promising therapy for metabolic syndrome.
The 11β-hydroxysteroid dehydrogenase (11β-HSD) enzymes, including 11β-HSD1 and 11β-HSD2, catalyze the inter-conversion between the inactive cortisone (11-dehydrocorticosterone in rodents) and the active cortisol in human (corticosterone in rodents) in a tissue specific manner (Fig. 1) [5]. 11β-HSD1 is highly expressed in key metabolic tissues, such as liver and adipose tissue. Transgenic over-expression of 11β-HSD1 in mice adipose tissue has caused visceral obesity, glucose intolerance, and insulin resistance [6, 7]. In obese patients, enhanced expression of 11β-HSD1 was also observed in adipose tissue and skeletal muscle [8, 9]. In contrast, global 11β-HSD1 knockout mice showed improved glucose tolerance, insulin sensitivity, and resistance to obesity when fed a high fat diet [6, 10,11,12]. On the other hand, 11β-HSD2 is predominately expressed in mineralocorticoid target tissues, such as the kidney and colon, which protects mineralocorticoid receptor from excessive activation by cortisol [13,14,15]. Evidence showed that inhibition of 11β-HSD2 would lead to sodium retention, hypokalaemia and hypertension [14, 16]. Accumulating evidence suggests that development of novel potent and selective 11β-HSD1 inhibitors is an attractive strategy for anti-diabetic therapy.
As a result, extensive efforts from the academic and industrial communities have been directed toward the discovery and development of selective 11β-HSD1 inhibitors [17,18,19]. Incyte, Merck, and others have demonstrated that 11β-HSD1 inhibitors improved glycemic control, lipid profiles, and blood pressure with modest weight loss [20]. However, none of them have progressed beyond phase 2 trials. Natural products (NPs), especially those derived from traditional Chinese medicines (TCMs), remain untapped sources of molecular scaffolds that can serve as lead compounds and potential therapeutic agents for diverse range of diseases (Fig. 2) [21].
Salvia miltiorrhiza Bunge, also known as the traditional Chinese medicine “Danshen”, has well-defined effects on cardiovascular and cerebrovascular disease. Extracts of S. miltiorrhiza roots in formulation of “Compound Danshen Dripping pill” are undergoing clinical trials in the United States. Up to now, more than 60 tanshinones have been isolated and identified from Danshen and related species [22]. Among them, tanshinone IIA and cryptotanshinone were the most studied because of their high abundance in plants and wide range of biological activities [23,24,25]. For instance, tanshinone IIA and cryptotanshinone have been shown to increase the activity of insulin by affecting tyrosine phosphorylation of the insulin receptor and activation of the down-stream kinase Akt, ERK1/2, and GSK3β [26] and ameliorated the diabetic complications [27,28,29]. We recently disclosed the discovery and SARs studies of tanshinones as novel indoleamine 2,3-deoxygenase (IDO1) and tryptophan 2,3-deoxygenase (TDO) dual inhibitors [30]. However, reports on their 11β-HSD1 inhibitory activities, as well as their structure–activity relationships, remain scarce, albeit that Park recently disclosed tanshinone IIA as a 11β-HSD1 inhibitor [31] following our patents on similar results being published in 2012 (CN 102304166A and CN 102603861A). As our constant efforts on developing naturally occurring therapeutic agents against type 2 diabetes [32, 33], we herein presented the development and structure–activity relationship studies of tanshinones as novel potent and selective 11β-HSD1 inhibitors.
2 Results
2.1 Discovery of tanshinones as novel 11β-HSD1 inhibitors
Our preliminary efforts towards the development of nature-derived 11β-HSD1 inhibitors led to the identification of eight active tanshinones (1–8) among a collection of natural products derived from Danshen and related species (Table 1). As shown in Table 1, most of them exhibited low nanomolar inhibitory activities against both human and mouse 11β-HSD1 but no detectable inhibition against 11β-HSD2. Remarkably, tanshinone IIA (1) and cryptotanshinone (2) exerted comparable inhibitory activities against 11β-HSD1 to that of the positive control (the nonselective 11βHSD inhibitor glycyrrhizic acid, GA), but with excellent selectivity over 11β-HSD2. Moreover, they are readily available. To improve the druggability and further explore their structure–activity relationships, a focused library of tanshinones by using 1 and 2 as the starting materials were thereby designed and synthesized.
2.2 Chemistry
2.2.1 Scaffold hopping design and synthesis
To elucidate the SARs of tanshinones, a scaffold hopping strategy was envisaged [34]. As shown in Scheme 1, several truncated tanshinone analogues 9–12 were designed. Basically, a “knockout strategy” was applied in designing analogues 9–12. For instance, compound 10 was envisaged by deletion of A-ring in tanshinone IIA (1). Further removal of B-ring generates compound 11. By comparisons of the biological profiles from 1 to 10 and 11 clearly illustrate the impacts of A- and B-ring on the the 11β-HSD1 inhibitory activities. In contrast, compounds 9 and 12 were designed to probe the role of ortho-quinone moiety. Analogues 9–12 were prepared from the commercially available starting materials according to the well-established protocols [35,36,37].
2.2.2 Modifications at A-ring
The chemical synthesis of tanshinone derivatives was depicted in Schemes 2, 3, 4, 5. As described previously [30], the modifications of A-ring commenced with the functionalization of C1 and C2 (Scheme 2). The installation of the alkene at C1, C2 moiety was achieved by reacting 1 with NBS in the presence of benzoyl peroxide, which delivered the alkene 13 in 53% yield, which was further converted to its derivatives via divergent pathways. For instance, SeO2-mediated oxidation of 13 in 1,4-dioxane delivered the corresponding α,β-unsaturated ketone 14 in 67% yield, which was further reduced to the C1, C2-saturated derivative 15 via catalytic hydrogenation. 15 was in turn transformed to the 3-acetoxy derivative 16 (89% yield) and 3-oxo derivative 17 (20% yield) by esterification and oxidation, respectively. In parallel, dihydroxylation of 13 with KMnO4 in the presence of HCOOH furnished 18a, 18b in 34%, 36% yields, respectively. The relative configuration of C1,C2-dihydroxy groups in 18a was assigned as trans by the JH1-H2 value (6.0 Hz) of its acetonide derivative of 18a [38]. Cationic reduction of 18a using tributyltin hydride in the presence of BF3.OEt2 gave the C2-OH derivative 19 in 87% yield. Alternatively, Swern oxidation of 18b delivered the enone 20 in 87% yield (Scheme 2).
Modifications on A-ring. Reagents and Conditions: a) NBS, Benzoyl peroxide, CCl4, reflux; b) SeO2, 1,4-dioxane, reflux; c) H2, Pd/C, MeOH/HCOOH (8:1), rt; d) Ac2O, Pyridine, DMAP, DCM, rt; e) PDC, Celite, DCM, rt; f) KMnO4, HCOOH, THF/H2O (4:1), rt; g) BF3.OEt2, n-Bu3SnH, DCM, 0 °C-rt; h) (COCl)2, DMSO, TEA, DCM, − 78 °C-rt
Modifications on C- and D-rings of 1. Reagents and Conditions: a) p-Hydroxybenzaldehyde, ammonium formate, AcOH, 100 oC; b) step 1: NIS, TFA, DCM, rt; step 2: ArB(OH)2, Pd(PPh3)4, PPh3, K2CO3, DMF / H2O (5:1), 80 °C; c) HCHO (aq.), HCl (con.), H2O, reflux; d) (HCHO)n, R1R2NH, AcOH, 110 °C; e) SeO2, AcOH, reflux; f) step 1: CBr4, PPh3, DCM, rt; step 2: PhNH2 or morpholine, Et3N, DCM, rt
2.2.3 Modifications at C- and D-rings
Scheme 3 depicts the chemical manipulations on the C- and D-rings of 2. The C11,C12-diacetoxy derivative 21 was obtained in 81% yield by catalytic hydrogenation of 2 in Ac2O in the presence of pyridine. Treatment of 2 with aq. NaOH (2.0 mol/L) with the assistance of ultrasound delivered (+)-neocryptotanshinone 22 in 91% yield [39], which was further oxidized to the corresponding aldehyde 23 in 65% yield. Subsequently, the pyrrol 24 was prepared by treatment of the aldehyde 23 with ammonia following the Paal–Knorr’s pyrroles synthesis [40]. On the other hand, treatment of 2 with aqueous ammonia provided the amine 25 in 50% yield [41] (Scheme 3).
On the other hand, modifications on the C- and D-rings of 1 were illustrated in Scheme 4. A three-component reaction between 1, p-hydroxybenzaldehyde and ammonium formate afforded the imidazole 26 in 48% yield [42]. To explore the effects of the C16-substituents on the 11β-HSD1 inhibitory activities and selectivity, a series of C16 functionalized derivatives were designed and synthesized. First, installation of an iodide at C16 by treatment of 1 with N-iodosuccinimide (NIS) in the presence of catalytic amount of trifluoroacetic acid delivered the desired product in 79% yield, which was followed by arylation under Suzuki’s protocol to afford the C16-aryl derivatives 27a–27e in 47–98% yields [43]. On the other hand, treatment of 1 with formalin in AcOH furnished the alcohols 28a and 28b. Similarly, a series of amines 29a–29d were also prepared by following the Pictet-Spengler’s protocol [35].
Derivatives with diverse functionalities at C17 were also designed and synthesized. Alder ene-type oxidation of the benzylic position with SeO2 afforded the alcohol 30 in 82% yield [44], which in turn enabled diverse further transformations. For instance, Corey-Fuch bromination delivered the bromide in 91% yield [45], which reacted with the amines in the presence of base to afford 31a and 31b in 81% and 92% yields, respectively.
2.2.4 Modifications at multi-positions
Based on the preliminary SARs studies, we envisaged to incorporate multiple privileged scaffolds into one molecular. As a result, a series of multiple position-modified derivatives were designed and synthesized using the privileged derivatives 13 and 30 as the starting materials (Scheme 5). On one hand, chlorination of 30 with N-chlorosuccinimide (NCS) furnished 32 in 97% yield. On the other hand, bromination of 13 with NBS delivered 33 in 74% yield. SeO2-mediated oxidation of 13 and 16 in AcOH delivered the alcohols 34 and 35 in 31% and 51% yields, respectively.
2.3 Selective 11β-HSD1 inhibition in vitro
With diverse tanshinones in hand, inhibition of human and mouse 11β-HSD1 enzymatic potency was measured by scintillation proximity assay (SPA) using microsomes containing 11β-HSD1 [46, 47]. Results are reported as the average of two or three independent experiments at each concentration. The preliminary evaluations of the derivatives were performed at the concentration of 1.0 μM (Table2). Derivatives with inhibitory ratio more than 50% in preliminary tests were further evaluated to calculate the IC50 value, as well as the selectivity ratio, against 11β-HSD2 (Table 3).
Based on the biological results shown in Tables 1, 2 and 3, the SARs of tanshinones were then discussed accordingly. For A ring, the cyclohexene moiety was superior to the aromatic one in term of the potency against both human and mouse 11β-HSD1 (i.e. 1 vs 6, 2 vs 5). Installation of alkene moiety at C1 and C2 exerted minor impacts on the activity (i.e. 13 vs 1). In contrast, polar groups at C1 and C2 significantly diminished inhibitory activity against human 11β-HSD1 but not mouse 11β-HSD1 (i.e., 18a–18b, 19, 20 vs 1). In addition, polar substituents at C3 and C4 (i.e., 3, 4, 14, 15–16) reduced the potency and selectivity against both human and mouse 11β-HSD1 relative to the lead compounds. Exceptionally, C3 ketone substituent exerted beneficial effects on the potency (i.e., 17 vs 1). Taken together, the hydrophilic substituent and proper flexibility of A-ring seems to be optimal for the potency, which is particularly true for human 11β-HSD1. Notably, results from scaffold hopping design by removing A ring revealed that A-ring seems to be not necessary for both activities and selectivity (i.e., 10 vs 1).
Interestingly, disrupting of ortho-quinone moiety led to a complete loss or a dramatic drop of activity against both human and mouse 11β-HSD1 (i.e., 7–8 vs 5, 21–22 vs 2, 26 vs 1), suggesting its critical role for the protein binding. Scaffold hoping results also supported this point (i.e., 9 vs 6, 12 vs 11).
It’s obvious that the D-ring opening derivatives exhibited significantly decreased potency against human 11β-HSD1, whereas the activities against mouse 11β-HSD1 retains for those preserving ortho-quinone moiety (i.e., 25 vs 2), but not for those with para-quinone moiety (i.e., 22). Surprisingly, the bioisosteres (i.e., 24 vs 1) exerted significantly reduced activities against both human and mouse 11β-HSD1. These results indicated that subtle changes in D-ring can dramatically affect the activity and selectivity against both human and mouse 11β-HSD1, which is particularly true for human 11β-HSD1. Variations at C16 also demonstrated interesting profiles. Derivatives containing C16 pyridinyl substituents (i.e., 27c), but not phenyl or quinolinyl ones (i.e., 27a-27e), produced comparable potency and selectivity to that of the parent compound 1. Moreover, derivatives with C16 hydroxymethyl or aminomethyl moieties (i.e., 28a, 28b and 29a) also demonstrated superior activities and excellent selectivity against both human and mouse 11β-HSD1. It’s noteworthy that C17 substituent also provided encouraging results. C17 hydroxyl derivative (i.e., 30) showed excellent activities and selectivity against both human and mouse 11β-HSD1, while other C17 derivatives (i.e., 31a and 31b) were much inferior. However, those with additional functionalities at D-ring (i.e., 32) resulted in a decrease in potency. Notably, derivatives 27d, 28b, 29a and 30 with polar groups not only exhibited excellent activities and selectivity, but also were with good druggability, which merit further investigations. Of particular note is that for the majority of the compounds listed in Table 3, their inhibitions against mouse 11β-HSD1 are more potent than that of human 11β-HSD1, which is in good agreement with the computational studies. Besides, the selectivity over mouse 11β-HSD2 is also better than that over human 11β-HSD2. Taken together, the SARs of tanshinones was summarized and illustrated in Fig. 3.
2.4 Molecular modeling analysis
To understand the structural basis for the binding affinity of the inhibitor for 11β-HSD1, we scrutinized the binding conformation of 1 by means of` molecular docking using PyMOL. Sequence alignment of human 11β-HSD1 with mouse 11β-HSD1 is shown in Figure S2. Critical different residues of the binding pocket for human and mouse 11β-HSD1 are illustrated in Figure S3. As shown in Fig. 4, the predicted 3D docking conformation of 1 in the mouse and human 11β-HSD1 binding site. The top-ranking docking score for 1 with mouse 11β-HSD1 is − 8.20 kcal/mol, and − 7.92 kcal/mol with human 11β-HSD1. This result shows that the binding affinity of 1 with mouse 11β-HSD1 is a little bit higher to human 11β-HSD1, which was also in good consistent with the experimental results, i.e., the IC50 value (43.89 nM) against human 11β-HSD1 is a little bigger than the IC50 value (13.29 nM) against mouse 11β-HSD1. There are two hydrogen bonds in the both systems which are formed between the C11-ketone in 1 and Tyr183 and Ser170, indicating C11-ketone as the key pharmacophore of tanshinone-based 11β-HSD1 inhibitors. This notion was further validated by the experimental results that interrupting the C-ring ortho-quinone moiety led to a dramatic drop of activities.
Three-dimensional (3D) interaction schemes between 1 and mouse/human 11β-HSD1. The figures were prepared using PyMOL (http://pymol.sourceforge.net/). The ligands and the residues near the pocket are shown as sticks (receptor carbon in cyan and ligand carbon in green). a Belongs to mouse 11β-HSD1 and b belongs to human 11β-HSD1. Hydrogen bonds are shown as dotted lines in 3D views
2.5 Cellular assay 11β-HSD1 inhibition in vitro
11β-HSD1 enzyme inhibition in adipose tissue is believed to be important for achieving efficacy in vivo [11, 12]. Derivatives with potent enzymatic potency and excellent selectivity profiles (13, 14, 17, 28a, 28b and 30) were also evaluated their 11β-HSD1 inhibition in 3T3-L1 adipocyte. As shown in Table 4, all of them showed moderate to good cellular efficacy. Among them, 1 and 17 exhibited comparable inhibitory activity against 11β-HSD1 to that of MK544, with IC50 of 2.0 μM and 0.4 μM respectively.
2.6 In vivo 11β-HSD1 inhibitory activities on ob/ob mice
After single oral administration of 1, 17 or 30 at dose of 300 mg/kg to ob/ob mice, the enzymatic activity of 11β-HSD1 in liver was measured. As shown in Fig. 5A, a significant decrease in 11β-HSD1 activity, by 32.9%, was observed in the liver of ob/ob mice at 4 h after treatment with 300 mg/kg of 1. As shown in Fig. 5B, the 11β-HSD1 activity were even more significantly decreased by 48.4%, 38.5% and 41.2% respectively upon 1, 17 or 30-administration after 8 h treatment, albeit that there is no significant difference between 1 and 17 or 30. These results revealed that 1, 17 and 30 exert potent and selective 11β-HSD1 inhibition both in vitro and in vivo.
3 Discussions and conclusion
TCM-derived natural products with unique skeletons and bioactivities remain a precious reservior for drug leads and candidates, thereby constituting a hotspot in the field of drug discovery. Tanshinone-based scaffold diversity was intensively investigated by incorporations of a series of novel “privileged” scaffolds, which significantly extended the chemical spaces around tanshinones. Furthermore, the general trends involving SARs of tanshinones were fully discerned from this focused diversity-oriented library and biological results. This work also led to the identification of a series of highly potent and selective 11β-HSD1 inhibitors. Among them, compounds 1, 17 and 30 were quite remarkable both in vitro and in vivo. Based on the biological results, systematic SARs were further investigated, which was also partially rationalized by a molecular docking model of 1 docked to the 11β-HSD1 based on the X-ray crystal structure of CBX bound to 11β-HSD1. This study not only provides a series of novel selective 11β-HSD1 inhibitors with promising therapeutic potentials in metabolic syndromes, but also expands the boundaries of the biological spaces of TCM-derived tanshinones. Further explorations of tanshinones in drug development are underway.
4 Experimental section
4.1 Chemistry
Procedures for the synthesis of the compounds mentioned in this article and their characterization data, as well as the spectra copies, are described in Supplementary Information.
4.2 Biological assay
4.2.1 11β-HSD1 and 11β-HSD2 inhibition assay
The SPA was used to screen for inhibitors of 11β-HSDs [48], with the microsome fractions prepared from the HEK-293 cells stably transfected with either human or mouse 11β-HSD1 or 11β-HSD2 as the enzyme source. Briefly, different concentrations of compounds were added to 96-well microtitre plates, followed by the addition of 80 μL of 50 mM HEPES buffer, pH 7.4 containing 25 nM [1,2-(n)3H]-cortisone and 1.25 mM NADPH (for 11β-HSD1 assay) or 12.5 nM [1,2,6,7-(n)3H]-cortisol and 0.625 mM NAD+(for 11β-HSD2 assay). Reactions were initiated by the addition of 11β-HSD1 or 11β-HSD2, enzyme. Procedure for the synthesis as microsome fractions from HEK293 cells in a final concentration of 320 μg/mL for human and mouse 11β-HSD1, 160 μg/mL for human 11β-HSD2 and 320 μg/mL for mouse 11β-HSD2, respectively. After a 60 min incubation at 37 °C, the reaction was stopped by the addition of 35 μL of 10 mg/mL protein A-coated yttrium silicate beads suspended in SuperBlock Blocking Buffer with 3 μg/mL of murine monoclonal cortisol antibody and 314 μM glycyrrhetinic acid. The plates were incubated under plastic film on an orbital shaker for 120 min at room temperature before counting. The amount of [3H]-cortisol generated in 11β-HSD1 enzyme reaction or remaining from the 11β-HSD2 enzyme reaction was captured by the beads and determined in a microplate liquid scintillation counter. The % inhibition was calculated relative to a non-inhibited control. Data were obtained from two or three independent experiments. IC50 values were calculated from concentration–response curves by a non-linear regression analysis using Prism Version 6.
4.2.2 11β-HSD1 activity assay in 3T3-L1 adipocytes
The reductase activity of 11β-HSD1 in intact 3T3-L1 adipocytes was determined by measuring the rate of conversion of cortisone to cortisol. To explore the effect of compounds on 11β-HSD1 reductase activity, 3T3-L1 adipocytes were incubated for 1 h at 37 ºC in serum-free DMEM containing 6.25 nmol/L [1,2-(N)3H]-cortisone and different concentrations of compounds, according to experimental design. 0.1% DMSO was set as the vehicle control and MK544 was set as the positive control. At the end of the incubation, 80 μL of medium was pipetted into a transparent bottom 96-well plate, and 35 μL of SuperBlock Blocking Buffer containing 10 g/L of protein A-coated yttrium silicate beads and 3 mg/L of anti-cortisol antibodies was added. The mixtures were shaken in the dark for 2 h and then used for liquid scintillation readings. The inhibition effect was calculated by the following equation:
4.2.3 Measurement of 11β-HSD1 activity in liver of ob/ob mice
B6.V-Lepob/Lepob mice (Jackson Laboratory, Bar Harbor, ME, USA) were bred at the Shanghai Institute of Materia Medica, Chinese Academy of Sciences. All mice were housed in a specific, pathogen-free class laboratory and were maintained at a controlled room temperature (22–24 °C) under a 12 h light–dark cycle with free access to water and food. The animal experiments were conducted in accordance with the guides by the Institutional Animal Care and Utilization Committee (IACUC) of Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences (CAS). The protocol was approved by the IACUC, SIMM, CAS with accreditation number of 2017-08-LY-67. All animal experiment complied with the ARRIVE guidelines for the care and use of laboratory animals.
Ob/ob mice (10 weeks’ old) were given a single dose of 1, 17, 30 (300 mg/kg) or vehicle (0.5% carboxymethyl cellulose, CMC), p.o. After 4 h and 8 h, animals were euthanized with an i.p injection of sodium pentobarbital (60 mg/kg) and the livers were isolated and stored as described above. The liver tissue was homogenized (1 mg/mL) in cold homogenization buffer, and 30 mg of liver homogenates was used to analyze the 11β-HSD1 activity by SPA, as previously described.
4.3 Computational methods
Tanshinone IIA (1) was prepared (generating stereoisomers and valid single 3D conformers) by means of the Ligand Procedure for the synthesis module in Maestro. The crystal structures of mouse and human 11β-HSD1 was retrieved from the Protein Data Bank (PDB entry: 1Y5R and 2IRW). All crystallographic water molecules were removed from the coordinate set. Cofactor NDP complexed in the crystal structure was reserved. Glide was used for docking. In the docking process, the standard docking score was used to rank the docking conformations. All the parameters were set as the default values. The grid-enclosing box was centered on the centroid of cocomplexed A in 11β-HSD1 and defined so as to enclose residues located within 14.0 Å around the A binding site and default van der Waal scaling was used (1.0 for the receptor and 0.8 for the ligand). Validation of the adopted docking procedure was conducted via re-docking of the co-crystallized ligands (corticosterone for 1Y5R and adamantane ether for 2IRW) and calculating RMSD between docked and re-docked ligands. The RMSD values between the top-scoring ligand orientation and the crystal ligand orientation 1Y5R and 2IRW were 0.33 and 0.65 Å, respectively, which were less than 2.0 Å (see Figure S1). The binding poses of tanshinone IIA (1) were modeled by Glide [49, 50] (Schrödinger 2010, Inc.) in SP mode with the same parameter settings.
References
Day C. Metabolic syndrome, or what you will: definitions and epidemiology. Diab Vasc Dis Res. 2007;4:32–8.
Tomlinson JW, Stewart PM. Mechanisms of disease: selective inhibition of 11β-hydroxysteroid dehydrogenase type 1 as a novel treatment for the metabolic syndrome. Nat Clin Pract Endocrinol Metab. 2005;1:92–9.
Wamil M, Seckl JR. Inhibition of 11B-hydroxysteroid dehydrogenase type 1 as a promising therapeutic target. Drug Discov Today. 2007;12:504–20.
Odermatt A. 11β-hydroxysteroid dehydrogenase type 1: a potential therapeutic target for the control of local glucocorticoid concentrations. Curr Enzyme Inhib. 2005;1:107–22.
Vantyghem MC, Marcelli-Tourvieille S, Defrance F, Wemeau JL. 11beta-hydroxysteroide dehydrogenases. Recent Adv Ann Endocrinol. 2007;68:349–56.
Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–70.
Masuzaki H, Yamamoto H, Kenyon CJ, Elmquist JK, Morton NM, Paterson JM, Shinyama H, Sharp MGF, Fleming S, Mullins JJ, Seckl JR, Flier JS. Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J Clin Invest. 2003;112:83–90.
Rask E, Walker BR, Söderberg S, Livingstone DEW, Eliasson M, Johnson O, Andrew R, Olsson T. Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11β-hydroxysteroid dehydrogenase type 1 activity. J Clin Endocrinol Metab. 2002;87:3330–6.
Kannisto K, Pietiläinen KH, Ehrenborg E, Rissanen A, Kaprio J, Hamsten A, Yki-Järvinen H. Overexpression of 11β-hydroxysteroid dehydrogenase-1 in adipose tissue is associated with acquired obesity and features of insulin resistance: studies in young adult monozygotic twins. J Clin Endocrinol Metab. 2004;89:4414–21.
Densmore VS, Morton NM, Mullins JJ, Seckl JR. 11β-hydroxysteroid dehydrogenase type 1 induction in the arcuate nucleus by high-fat feeding: a novel constraint to hyperphagia? Endocrinology. 2006;147:4486–95.
Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CRW, Seckl JR, Mullins JJ. 11β-Hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci. 1997;94:14924–9.
Eijnde BO, Van LM, Brouns F, Van DVGJ, Labarque V, Ramaekers M, Van SR, Verbessem P, Wijnen H, Hespel P. No effects of oral ribose supplementation on repeated maximal exercise and de novo ATP resynthesis. J Appl Physiol. 1985;91(2001):2275–81.
Nair S, Lee YH, Lindsay RS, Walker BR, Tataranni PA, Bogardus C, Baier LJ, Permana PA. 11β-Hydroxysteroid dehydrogenase Type 1: genetic polymorphisms are associated with Type 2 diabetes in Pima Indians independently of obesity and expression in adipocyte and muscle. Diabetologia. 2004;47:1088–95.
Kotelevtsev Y, Brown RW, Fleming S, Kenyon C, Edwards CRW, Seckl JR, Mullins JJ. Hypertension in mice lacking 11β-hydroxysteroid dehydrogenase type 2. J Clin Invest. 1999;103:683–9.
Wilson RC, Dave-Sharma S, Wei J-Q, Obeyesekere VR, Li K, Ferrari P, Krozowski ZS, Shackleton CHL, Bradlow L, Wiens T, New MI. A genetic defect resulting in mild low-renin hypertension. Proc Natl Acad Sci. 1998;95:10200–5.
Walker BR, Connacher AA, Lindsay RM, Webb DJ, Edwards CRW. Carbenoxolone increases hepatic insulin sensitivity in man: a novel role for 11-oxosteroid reductase in enhancing glucocorticoid receptor activation. J Clin Endocrinol Metab. 1995;80:3155–9.
Boyle CD, Kowalski TJ, Zhang L. 11beta -hydroxysteroid dehydrogenase type 1 inhibitors. Ann Rep Med Chem. 2006;41:127–40.
Scott JS, Goldberg FW, Turnbull AV. Medicinal chemistry of inhibitors of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). J Med Chem. 2014;11:4466–86.
Hale C, Wang M. Development of 11 beta-HSD1 inhibitors for the treatment of type 2 diabetes. Mini-Rev Med Chem. 2008;8:702–10.
Anderson A, Walker BR. 11β-HSD1 inhibitors for the treatment of type 2 diabetes and cardiovascular disease. Drugs. 2013;73:1385–93.
Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–35.
Gong L, Haiyu X, Wang L, Xiaojie Y, Huijun Y, Songsong W, Cheng L, Ma X, Gao S, Liang R, Yang H. Identification and evaluation of the chemical similarity of Yindan xinnaotong samples by ultra high performance liquid chromatography with quadrupole time-of-flight mass spectrometry fingerprinting. J Sep Sci. 2016;39:611–22.
Gao S, Liu Z, Li H, Little PJ, Liu P, Xu S. Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis. 2012;220:3–10.
Tian X-H, Wu JH. Tanshinone derivatives: a patent review (January 2006–September 2012). Exp Opin Therap Pat. 2013;23:19–29.
Xu S, Liu P. Tanshinone II-A: new perspectives for old remedies. Exp Opin Therap Pat. 2013;23:149–53.
Jung SH, Seol HJ, Jeon SJ, Son KH, Lee JR. Insulin-sensitizing activities of tanshinones, diterpene compounds of the root of Salvia miltiorrhiza Bunge. Phytomedicine. 2009;16:327–35.
Xie Z, Loi TT, Zhang P, Xu F, Xu X, Li P. Dan-Qi prescription ameliorates insulin resistance through overall corrective regulation of glucose and fat metabolism. J Ethnopharmacol. 2015;172:70–9.
Li X. Effect of tanshinone II A combined with atorvastatin calcium on plasma lipid peroxidation and IL-6 levels in type 2 diabetes patients with macroangiopathy. Wuhan Daxue Xuebao, Yixueban. 2011;32:800–2.
Chen Y, Li C, Cai F. Effect of sodium tanshinone IIA sulfonate(A) on SIRT1 and Fox01 in kidney of diabetic rats. Zhongyao Yaoli Yu Linchuang. 2015;31:47–50.
Liu J, Ren J, Yang K, Chen S, Yang X, Zhao Q-S. Discovery and biological evaluation of tanshinone derivatives as potent dual inhibitors of indoleamine 2, 3-dioxygenase 1 and tryptophan 2, 3-dioxygenase. Eur J Med Chem. 2022;235: 114294.
Park SB, Park JS, Jung WH, Park A, Jo SR, Kim HY, Rhee SD, Ryu SY, Jeong HG, Park S, Lee H, Kim KY. Identification of a novel 11β-HSD1 inhibitor from a high-throughput screen of natural product extracts. Pharmacol Res. 2015;102:245–53.
Deng X, Shen Y, Yang J, He J, Zhao Y, Peng L-Y, Leng Y, Zhao Q-S. Discovery and structure–activity relationships of ent-Kaurene diterpenoids as potent and selective 11β-HSD1 inhibitors: potential impact in diabetes. Eur J Med Chem. 2013;65:403–14.
Chen X-Q, Shao L-D, Pal M, Shen Y, Cheng X, Xu G, Peng L-Y, Wang K, Pan Z-H, Li M-M, Leng Y, He J, Zhao Q-S. Hupehenols A-E, selective 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitors from Viburnum hupehense. J Nat Prod. 2015;78:330–4.
Hu Y, Stumpfe D, Bajorath J. Recent advances in scaffold hopping. J Med Chem. 2017;60:1238–46.
Bian J, Deng B, Xu L, Xu X, Wang N, Hu T, Yao Z, Du J, Yang L, Lei Y, Li X, Sun H, Zhang X, You Q. 2-Substituted 3-methylnaphtho[1,2-b]furan-4,5-diones as novel L-shaped ortho-quinone substrates for NAD(P)H:quinone oxidoreductase (NQO1). Eur J Med Chem. 2014;82:56–67.
Bian J, Li X, Wang N, Wu X, You Q, Zhang X. Discovery of quinone-directed antitumor agents selectively bioactivated by NQO1 over CPR with improved safety profile. Eur J Med Chem. 2017;129:27–40.
Ghosh K, Karmakar R, Mal D. Total synthesis of neo-tanshinlactones through a cascade benzannulation-lactonization as the key step. Eur J Org Chem. 2013;2013:4037–46.
Rousseau J-F, Dodd RH. Synthesis of 3-deaza-β-hydroxyhistidine derivatives and their use for the preparation of substituted pyrrolo[2,3-c]pyridine-5-carboxylates via the Pictet−Spengler reaction. J Org Chem. 1998;63:2731–7.
Marrero JG, Andrés LS, Luis JG. Quinone derivatives by chemical transformations of 16-hydroxycarnosol from Salvia species. Chem Pharm Bull. 2005;53:1524–9.
Minetto G, Raveglia LF, Taddei M. Microwave-assisted Paal−Knorr reaction. A rapid approach to substituted pyrroles and furans. Org Lett. 2004;6:389–92.
An L-K, Bu X-Z, Wu H-Q, Guo X-D, Ma L, Gu L-Q. Reaction of tanshinones with biogenic amine metabolites in vitro. Tetrahedron. 2002;58:10315–21.
Lin W, Yuan L, Tan W, Feng J, Long L. Construction of fluorescent probes via protection/deprotection of functional groups: a ratiometric fluorescent probe for Cu2+. Chem - Eur J. 2009;15:1030–5.
Miyaura N, Suzuki A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem Rev. 1995;95:2457–83.
Gong W, Liu Y, Zhang J, Jiao Y, Xue J, Li Y. Regio- and stereoselective [4+3] cycloaddition towards fused 5,7,6-tricyclic skeletons. Chem Asian J. 2013;8:546–51.
Butini S, Campiani G, Borriello M, Gemma S, Panico A, Persico M, Catalanotti B, Ros S, Brindisi M, Agnusdei M, Fiorini I, Nacci V, Novellino E, Belinskaya T, Saxena A, Fattorusso C. Exploiting protein fluctuations at the active-site gorge of human cholinesterases: further optimization of the design strategy to develop extremely potent inhibitors. J Med Chem. 2008;51:3154–70.
Mundt S, Solly K, Thieringer R, Hermanowski-Vosatka A. Development and application of a scintillation proximity assay (SPA) for identification of selective inhibitors of 11β-hydroxysteroid dehydrogenase type 1. Assay Drug Dev Technol. 2005;3:367–75.
Solly K, Mundt SS, Zokian HJ, Ding GJ-F, Hermanowski-Vosatka A, Strulovici B, Zheng W. High-throughput screening of 11β-hydroxysteroid dehydrogenase type 1 in scintillation proximity assay format. Assay Drug Dev Technol. 2005;3:377–84.
Mundt S, Solly K, Thieringer R, Hermanowski-Vosatka A. Development and application of a scintillation proximity assay (SPA) for identification of selective inhibitors of 11 beta-hydroxysteroid dehydrogenase type 1. Assay Drug Dev Techn. 2005;3:367–75.
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–49.
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem. 2004;47:1750–9.
Acknowledgements
We thanked professor Wei-Liang Zhu for precious advices. We also thanked the National Natural Science Foundation of China (No. U1502223), Hunan Provincial Key Research and Development Project (Grant No. 2021WK2005 to X. Deng), Natural Science Foundation of Hunan Province (Grant No. 2021JJ30894 to X. Deng) and the open fund of State Key Laboratory of Phytochemistry and Plant Resource in West China (Grant No. P2020-KF03).
Author information
Authors and Affiliations
Contributions
QZ and YL contributed to the project conception/supervision, funding acquisition. XD, JR, ZP and HZ carried out the isolation, design and synthesis of tanshinones. SH, YS carried out the biological evaluations. JY and ZZ performed the computational studies. XD, SH, JR, ZP and JY drafted the manuscript. QZ, YL and ZZ reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare no conflict of interest.
Research involving human participants and/or animals and informed consent
This article does contain animal studies, but not involve human participants. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deng, X., Huang, SL., Ren, J. et al. Development and structure–activity relationships of tanshinones as selective 11β-hydroxysteroid dehydrogenase 1 inhibitors. Nat. Prod. Bioprospect. 12, 36 (2022). https://doi.org/10.1007/s13659-022-00358-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13659-022-00358-9