Abstract
More than 30 years after the first infestation report on Apis mellifera, varroa mites are still damaging honey bee health. The present paper reports data from a field experiment concerning two early spring (late February) bio-technical management techniques: early brood interruption (through queen caging) and early trapping comb (through the use of a single trapping comb). The aim of the study was to evaluate the impact of both techniques on colony development, mite population growth and honey production during the 6 months following the treatments. We observed that the early season queen handling and technical management of the colonies were able to affect mite reproduction resulting in a lower infestation rate, especially in the brood interruption group, while not causing any repercussion on the honey harvest and seasonal colony development and performance.
Similar content being viewed by others
1 Introduction
Honey bee colony losses are a complex phenomenon recently occurring on a large scale: first described in the USA as CCD or colony collapse disorder (Underwood and van Engelsdorp 2007; vanEngelsdorp et al. 2009), the widespread depopulation of bee hives was then observed in Europe and globally (Mutinelli et al. 2010; Lee et al. 2015). Possible drivers for colony losses are the single or synergistic effect of pesticides, infectious diseases, parasitic mite infestations, climate change, poor nutritional sources and beekeeping practices (Maini et al. 2010). Among these, the ectoparasitic mite Varroa destructor (Anderson and Trueman 2000), which has spread globally during the past 50 years, has resulted in the death of millions of honey bee (Apis mellifera, Linnaeus 1758) colonies, although its role in colony death has not been completely clarified (Martin 2001).
The spread of mites has facilitated the spread of viruses (Martin et al. 2012) by acting as a viral reservoir and incubator (Gisder et al. 2009). In addition, the mites’ feeding behaviour allows viruses to be transmitted directly into the bees’ haemolymph, thus bypassing established oral and sexual routes of transmission (Martin et al. 2012; Amiri et al. 2015; Ravoet et al. 2015).
The global scientific interest in the varroa mite is well represented within the international honey bee research network COLOSS (http://www.coloss.org/), where two specific task forces, ‘varroa control’ and ‘survivors’, are active to achieve a full understanding and hopefully to solve the problem. The current knowledge on the interactions between the varroa mite, its environment, and the honey bee host have been recently described by Nazzi and Le Conte (2016), Dolezal et al. (2016) and Ramsey and van Engelsdorp (2016).
From a practical perspective, during the last decade, substantial progress has been made: a wide range of different chemical and organic active ingredients, formulations and application techniques to reduce mite populations have been registered and made available to beekeepers; however, the varroa mite has still a remarkable effect on beekeeping and feral honeybee populations, given that none of the control strategies are completely effective or free from side effects for honeybees (Rosenkranz et al. 2010) and in large areas honey bee colonies struggle to survive in the wild (Jaffé et al. 2010). Mite infestation thus constitutes a persistent threat which may lead to weakening and loss of colonies as soon as failure of the treatment strategies occurs (Lodesani and Costa 2005). Moreover, years of systematic treatments with synthetic acaricides applied a selective pressure on mite populations, leading to resistance to some of the drugs. Resistance to acaricides is a serious problem in chemotherapy for V. destructor and can cause disastrous colony losses if control of the mite relies on ineffective treatment (Lodesani et al. 1995; Baxter et al. 1998; Elzen et al. 1998; Lodesani and Costa 2005; Berry et al. 2013). If the inter-treatment intervals are prolonged as long as possible, this not only reduces net chemical use with its hazards to bees, honey and the environment, but enables mites through genetic recombination and reproduction over time to conserve their chemical susceptible genes, thus prolonging the useful life of an acaricide (Metcalf 1982).
Many scientists working in varroa control agree that bio-mechanical control methods and chemical substances application can complement each other (Rosenkranz et al. 2010; Nanetti et al. 2012, 2016; Gregorc et al. 2017). In Italy, bio-technical measures, such as trapping comb or queen caging, are becoming widespread (Lodesani et al. 2014) as the high winter colony losses reported in recent years seem to point to inefficacy of some conventional chemical treatments to control varroa mite infestation (Mutinelli et al. 2010).
The mechanical interruption of the mites’ reproductive cycle, or the removal of a significant number of mites, can be achieved with a number of different techniques including the periodical use of a ‘trapping comb’ through the season, the complete trapping comb or queen caging for a complete brood cycle (Maul et al. 1988; Besana et al. 2010), for which different models of cages are now available. These bio-technical measures are also used in combination among each other and with treatment with organic acids (Nanetti et al. 2011).
Despite the wide acceptance among beekeepers, some issues, such as the time-demanding task to find the queen in a fully developed colony and the contemporaneity of treatment with the summer honey harvest, still represent a problem for some of them.
To provide beekeepers with further flexibility in the seasonal mite control strategy, in the present study, we separately evaluated two bio-mechanical management techniques (trapping comb and brood interruption) on honey bee colonies in early spring with the following aims: (1) to verify the effectiveness in reducing the mite infestation; (2) to assess the impact of these practices on the future development of the colonies and on the total honey harvest through the whole season.
2 Material and methods
2.1 Test hives constitution and artificial mite infestation
A total of 30 Apis mellifera ligustica (Spinola, 1806) colonies were bought from a single-bee breeder member of the “National Register of Queen Bee Breeders” and moved to the same beeyard in Reggio Emilia (Pianura Padana, northern Italy) for testing. Honey bee queens were all from the same genetic line and mated in a single mating station the same year.
In September, colony strength was balanced according to the Liebefeld method (Imdorf et al. 1987) by two operators as recommended by Delaplane et al. (2013), and an empty frame carrying a Var-Control Cage Mozzato model (Figure 1) was introduced in each colony. In October, queens were caged for 22 days (no drone brood was present), and a treatment by trickling an oxalic acid solution (100 g oxalic acid dihydrate, 1000 g sucrose and 1 L water) was performed at the end of caging period to remove varroa mites. The individual infestation level for each colony was confirmed by sugar shaking adult bees (according to Dietemann et al. 2013), 1 week after treatment, and no mites were found.
The first week of November, 8 days after the treatment, live adult female varroa mites were collected using the sugar shaking method from a single-donor colony from a different beeyard (a modification of method described by Lodesani et al. (2002)); the same day, all of the 30 test hives were artificially infested, adding manually 15 mites each; colonies were then left untouched and untreated for the whole overwintering period.
2.2 Test groups, beeyard constitution and treatments
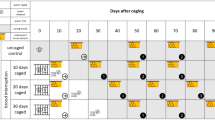
At the end of February of the next year, the colonies were inspected and the worker bee population size was visually estimated as previously described; the colonies were split into three homogeneous groups according to bee population and honey stores: ‘C’ (control), ‘S’ (queen caging) and ‘R’ (trapping comb) of 10 units each; five colonies from each group were then moved to a second test location located approximately 600 m away, as safety measure with respect of possible late-season mite spillover. Honey bee queens were either caged in a Var-Control cage Mozzato model (group ‘S’) or in a full frame cage GB model (group ‘R’) (Figure 1). Hives from the ‘C’ group were inspected: queens were collected and released to perform the same handling within groups. Twenty-one days later, Var-Control cages (group ‘S’) were opened and GB cages (group ‘R’) were removed altogether with the single isolated brood frame, which acted as a trapping comb (this was frozen at – 20 °C and later inspected to quantify mite presence). Oxalic acid treatment was performed in ‘R’ and ‘S’ groups with identical formulation and application as past October. At cage opening/removal, adhesive sheets were inserted on the hives’ bottom boards.
2.3 Longevity assay
Each colony of the three groups was sampled for adult worker bees collected from external honey combs, at day 19 of the caging period to perform survival test under lab condition; 20 bees/colony were moved into small wooden, glass-sided cages measuring 10 × 10 × 20 cm, and kept at 33 °C, 70% RH, in the dark; cages were supplied with gravity feeders and bees were fed ad libitum with 50% w/v sucrose solution. Individual mortality was recorded daily for the following 4 weeks.
2.4 Post treatment evaluation
All hives were inspected to estimate colony development by scoring the amount of bees, brood cells, pollen stores and honey stores (Imdorf et al. 1987; Delaplane et al. 2013) respectively at 19, 41, 67, 96 and 119 days after treatment.
Total honey harvest was measured by weighing supers before and after honey extraction for every single colony. Being the late spring ‘acacia’ (Robinia pseudoacacia L.), honey, an important harvest for most of the Italian beekeepers, weight values from acacia supers were also recorded separately. The last supers were removed in late July, and 2 days later, all queens from ‘C’, ‘S’ and ‘R’ groups were caged in a Var-Control cage Mozzato model (Figure 1); a trickling oxalic acid treatment (the same as used at the beginning of the experiment) was performed 25 days after caging during the queen releasing, and the total number of fallen mites was scored by the use of adhesive bottom sheets for 3 weeks after treatments. Time schedule is shown in Figure 2.
2.5 Statistical analysis
Normal distribution of population size within groups was assessed with Shapiro-Wilk’s test; homoscedasticity of distribution was assessed using Levene’s test. Significant differences in population size among groups (adult bees and brood cells number), nest stores (honey cells and pollen cells number), acacia, and total honey harvest and total mites were tested with a one-way ANOVA analysis. To establish differences among different groups, Bonferroni multiple comparison procedure was used at the P = 0.05 significance level. Results are expressed as mean ± SE.
Overall differences in longevity among groups were tested for significance with the Kaplan-Meier survival test, while paired significance comparisons between groups were evaluated by a Log-Rank test. Repeated measurement ANOVA was used to test significant differences for each of the abovementioned parameters against group and location factors, while two-way ANOVA analyses were used to test significant differences with respect of any time point. All reported analyses were carried out using Statistica-StatSoft v. 7.1 (Statsoft, Inc. 2008).
3 Results
3.1 Bee longevity
Average longevity of caged bees under lab conditions was 22.7 ± 1.1 days for group ‘C’, 24.5 ± 0.9 days for group ‘S’ and 25.0 ± 0.9 days for group ‘R’. Survival curve analysis (Figure 3) performed with Kaplan-Meier showed highly significant differences among groups (chi-squared 68.73, P < 0.01) with longevity of ‘R’> ‘S’ > ‘C’ groups; log-rank paired analyses between groups also provided highly significant differences among ‘C’ versus ‘S’ and ‘C’ versus ‘R’ comparisons (respectively t = 6.30, P < 0.01 and t = 8.40, P < 0.01); group ‘S’ versus group ‘R’ comparison was also significantly different with P < 0.05 (t = 2.26).
3.2 Mite infestation and post treatment colony development
At the beginning of the field test, the average number of worker bees per colony within group was in ‘C’ = 6300 ± 598, in ‘S’ = 6200 ± 290 and in ‘R’ = 6625 ± 274. Population size within ‘C’, ‘S’ and ‘R’ groups showed normal distribution according to Shapiro-Wilk’s test, each group respectively scoring W = 0.9571; 0.875; 0.9291 (P > 0.05 for all); one-way ANOVA analyses showed non-significant differences (F = 0.286, P > 0.05) among groups. The average number of mites fallen after the oxalic acid treatment performed at the end of February was 5.3 ± 1.14 in ‘S’ group and 3.6 ± 1.01 in ‘R’ group. Differences between groups were not statistically significant (F = 0.80, P > 0.05). In the ‘R’ group, an average of 2680 ± 400 (min 800, max 4000) brood cells per colony was removed with an average infestation level of 0.082 ± 0.035.
In Figure 4, the average amount of adult bees, brood cells, pollen stores and honey stores for each group is shown. Repeated measurement ANOVA failed to highlight any statistically significant difference for bees’ number (F = 0.689, P > 0.05), brood cells (F = 0.421, P > 0.05), honey (F = 0.540, P > 0.05) and pollen (F = 0.793, P > 0.05) stores. Two-way ANOVA analyses showed significant differences among groups at t = 19 for both number of adult bees and brood cells number (respectively F = 3.461, P < 0.05 and F = 4.27, P < 0.05), with ‘C’ having more bees and brood than both ‘S’ and ‘R’. About the amount of honey stores in the nest, the initial number of honey cells stored per colony within group was assessed in ‘C’ = 17,000 ± 5164, ‘S’ = 15,000 ± 5164 and ‘R’ = 13,500 ± 5000. One-way ANOVA analyses showed non-significant differences (F = 4.692, P > 0.05) among groups. Significant differences were scored using Two-way ANOVA at t = 19 (F = 5.253, P < 0.05) where ‘R’ group had lower amount of both ‘C’ and ‘S’ groups; at t = 41, ‘C’ group had higher honey stores respect to both ‘R’ and ‘S’ groups (F = 4.064, P < 0.05). No significant differences in amount of pollen stores among groups were observed at any of the time points.
Seasonal development of bees (1), brood (2), honey (3) and pollen (4) cells. Total number (vertical axis) is measured at different days (horizontal axis) from the treatment. Repeated measurement ANOVA did not score any statistically significant difference. Asterisks highlight statistically significant difference at a peculiar time point according to a two-way ANOVA.
The strength of the colonies at the end of the experiment (late July) during the queen caging operation expressed as number of brood cells was: C = 35,520 ± 1932, S = 37,680 ± 1604, R = 40,400 ± 1631. One-way ANOVA showed non-significant difference (F = 0.49, P > 0.05) in the final strength of the colonies thus measured.
The mean number of total brood cells reared by the colonies of each group (as sum of the total brood cells of each measurement by every colony) during the whole test period is reported in Table I. Differences among the groups were not statistically significant (One-way ANOVA F = 0.491, P > 0.05).
3.3 Honey production
The average total honey production in the two test apiaries was 49.60 ± 4.77 kg and 47.28 ± 5.49 kg; the average total honey production was 47.55 ± 6.02 kg, 46.80 ± 4.39 kg and 51.62 ± 5.78 kg respectively for ‘C’, ‘S’ and ‘R’ groups (Table I). Score differences were analysed through a two-way ANOVA and resulted not significant for both factors (location F = 0.1259, P > 0.05; group F = 0.1935, P > 0.05). The same analysis was performed on the share of acacia honey: ‘C’ group produced 10.5 ± 1.94 kg, ‘S’ group 8.3 ± 2.46 kg and ‘R’ group 11.0 ± 1.75 kg on average. Again, none of the factors involved showed any significant difference (location F = 0.083, P > 0.05; group F = 0.4542, P > 0.05).
3.4 Final varroa mite infestation level
The mean level of infestation in the three groups at the end of the period was significantly different, with 1846 ± 354 mites per colony in ‘C’ group, 725 ± 117 in ‘S’ group and 1417 ± 321 in ‘R’ group (Figure 5) (one-way ANOVA: F = 4.154, P < 0.05). Post hoc Bonferroni test showed a significant difference between ‘C’ and ‘S’ groups (P < 0.05).
4 Discussion
Recently, many authors (Nanetti et al. 2012, 2016; Pietropaoli et al. 2012; Lodesani et al. 2014; Gregorc et al. 2017) have shown that combining oxalic acid application with an artificial broodless condition is an alternative and/or effective management strategy for varroa mite control during the harvest season. In this study, we demonstrated that egg-laying suppression is more effective to reduce mite infestation than trapping comb, when applied in early spring. Moreover, under laboratory condition, both brood interruption and trapping comb boost honey bee life span. The significantly increased longevity detected in ‘S’ and ‘R’ groups might be attributed to the diminished work load for those bees (reduced brood care in comparison to the ‘C’ group) (Amdam et al. 2009); we also detected a longer lifespan for the ‘R’ group with respect to the ‘S’ group and this might be explained by the ‘trapping comb’ effect, diminishing the mites’ load on adult bees during the treatment, thus preventing some further damage on adult bees; on a deeper look, the difference among ‘R’ and ‘S’ groups is not significant if we stop the analyses at day 20 (results not shown): speculation can be made on a cumulative effect due to viral disease, which might be enhanced in very old worker bees, but further investigation and specific assays would be needed to verify this hypothesis.
The low infestation rate scored in the removed trapping comb group (‘R’) is consistent with our previous results (unpubl. data). However, the difference in final infestations does not seem justified by the few mites removed during the treatments; probably, we should consider the “disturbance” caused to the survived mites by the bio-technical measures applied just before their first reproduction opportunity, after the winter pause. Some unknown factors other than those already known could have induced chronic or sublethal effect in the survivor mites: the synergic effect of prolonged absence of receptive brood and the presence of oxalic acid residues could have caused detrimental effect on the reproduction cycle of the survived mites (personal communication, Nanetti 2017). Other factors like the diminished mating attitude for mites during the very early spring (Al Ghzawi 1992) and prevalence of incestuous mating at the beginning of the brood season (Beaurepaire et al. 2017) could be somehow involved in the reduced population growth of the survived mite population.
Queen caging (‘S’ group) resulted the one with the lowest final infestation level.
The significantly lower amount of bees and brood scored at day 19 in the ‘S’ and ‘R’ with respect to the ‘C’ groups was expected: both bio-techniques strongly affect colony development at the initial stage, thus strengthening the robustness of post treatment data. Indeed, at about 40 days after treatment, colonies in the treated groups had fully recovered size and strength and had a larger amount of brood cells after day 41 (Figure 4). This difference, combined with the increased lifespan of bees in the treated groups, could be explained with an effective sanitization of the brood that might further increase the late season harvesting potential for those colonies. The total amount of brood reared by the colonies in the whole test period clearly indicates that the colonies were able to recover the initial gap.
Despite being already widely accepted among Italian beekeepers, artificial brood interruption methods to increase the efficacy of treatment with organic compounds against varroa mite infestation are still debated due to supposed counter side effects, such as a greater time consumption and a reduced honey harvest. We did not score the average time needed to find and cage the queen, but it is safe to say that the queen-finding operation in early spring is faster than in a fully developed colony. Our results also disprove the scepticism with respect to diminished honey harvest: the acacia harvest, the first and most important one in most of the Italian peninsula, was obtained with no significant difference with respect to the control group. In our experimental area, acacia is blooming early May, thus about 9 weeks post treatment. Our results showed that two complete brood cycles are needed to recover the bee/brood population and 20 extra days (consistent with the time needed for a newly emerged honey bee to develop into a forager) to fully recover the harvest potential of the colony. Furthermore, the total honey production confirms that the early season queen handling and the technical management of the colonies do not cause any repercussion on the future colony development and performance.
In this study, where great effort was put to start the experiment with comparable groups according to genetic origin, initial colony strength and mite infestation level, the early use of the trapping comb or the early queen caging provides an alternative strategy for varroa control especially for honey-oriented beekeeping companies that rely also on summer and late summer harvests. Our observation stopped at the end of July (that more or less coincides with the end of the blooming season in the north of Italy), but the trends of the parameters we scored suggest also a higher potential for the remainder of the summer season.
Mid-February turned out a valuable time frame for IPM through brood management in our testing area, (Pianura Padana) since the effort for queen finding was reduced thanks to the small colony size after winter and honey harvest was not affected: we underline the importance of shifting the treatment time according to the different climates, suggesting late January as more appropriate for Mediterranean regions (where bees have almost no natural winter brood interruption and citrus trees that provide an important harvest are blooming in April) and early March for apiaries located in the Alps and Apennines over 500 m a.s.l.
We recommend beekeepers to consider this method as an alternative in the annual varroa control strategy, to avoid surprises during the harvest season due to ineffective treatments of the previous year or robbing and drift episodes in the fall (quite common in A. m. ligustica race). Starting the harvest season with a low and a more uniform infestation level in the apiary is a warranty to maintain healthy colonies (there were less than half the mites in the brood interruption group than in the control) and to reach the end of the season without any other intervention. Further studies are necessary to investigate the possibility of intervening in the season only at the very beginning and in the late fall, thus avoiding to interfere with the colonies during the harvest season.
References
Al Ghzawi, A. (1992) Factors affecting the maturity of young females of Varroa jacobsoni Oudemans. PhD Thesis, University of Hohenheim
Amdam, G. V., Rueppell, O., Fondrk, M. K., Page, R. E., & Nelson, C. M. (2009). The nurse’s load: early-life exposure to brood-rearing affects behavior and lifespan in honey bees (Apis mellifera). Exp. Gerontol. 44(0), 467–471. https://doi.org/10.1016/j.exger.2009.02.013
Amiri, E., Meixner, M., Nielsen, S.L., Kryger, P. (2015) Four categories of viral infection describe the health status of honey bee colonies. PLoS One 8, 2015 https://doi.org/10.1371/journal.pone.0140272
Anderson, D.L., Trueman, J.W.H. (2000) Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 24, 165–189
Baxter, J., Eischen, F., Pettis, J., Wilson, W.T., Shimanuki, H. (1998) Detection of fluvalinate-resistant Varroa mites in U.S. honey bees. Am. Bee J. 138, 291.
Beaurepaire, A. L., Krieger K. J, Moritz, R. F. A. (2017) Seasonal cycle of inbreeding and recombination of the parasitic mite Varroa destructor in honeybee colonies and its implications for the selection of acaricide resistance. Infect. Genet. Evol. 50, 49–54.
Berry, J.A., Hood W. M., Pietravalle, S., Delaplane, K.S. (2013) Field-level sublethal effects of approved bee hive chemicals on honey bees (Apis mellifera L). PLoS One 8, 10, e76536 https://doi.org/10.1371/journal.pone.0076536
Besana, A., Baracani, G., Aureli, S., Galuppi, R., Tampieri, M., Nanetti, A. (2010) Oxalic acid associated to queen caging and Api Life Var in the summer treatment of Varroa destructor infesting the honey bee colonies. In Atti: 26° Congresso Nazionale della Società Italiana di Parassitologia, Perugia, 22 - 25 Giugno 2010.
Delaplane, K. S., Van Der Steen, J., Guzman, E. (2013) Standard methods for estimating strength parameters of Apis mellifera colonies. In Dietemann, V; Ellis, J D; Neumann, P (eds) The COLOSS BEEBOOK, Volume I: standard methods for Apis mellifera research. J. Apic. Res. 52(1). https://doi.org/10.3896/IBRA.1.52.1.03
Dietemann, V., Nazzi, F., Martin, S. J., Anderson, D., Locke, B., Delaplane, K. S., Wauquiez, Q., Tannahill, C., Frey, E., Ziegelmann, B., Rosenkranz, P., Ellis, J. D. (2013) Standard methods for varroa research. In Dietemann, V; Ellis J D; Neumann P (eds) The COLOSS BEEBOOK, Volume II: standard methods for Apis mellifera pest and pathogen research. J. Apic. Res. 52(1). https://doi.org/10.3896/IBRA.1.52.1.09
Dolezal, A.G., Carrillo-Tripp, J., Miller, W.A., Bonning, B.C., Toth, A.L. (2016) Intensively cultivated landscape and varroa mite infestation are associated with reduced honey bee nutritional state. PLoS One 11(4), e0153531. https://doi.org/10.1371/journal.pone.0153531
Elzen, P.J., Eischen, F.A., Baxter, J.R., Pettis, J., Elzen, G.W., Wilson, W.T. (1998) Fluvalinate resistance in Varroa jacobsoni from several geographic locations. Am. Bee J. 138, 674–676
Gisder, S., Aumeier, P., Genersch, E. (2009) Deformed wing virus: replication and viral load in mites (Varroa destructor). J. Gen. Virol., 90(Pt 2), 463–7. https://doi.org/10.1099/vir.0.005579-0.
Gregorc, A., Alburaki, A., Werle, C., Knight, P.R., Adamczyk, J. (2017) Brood removal or queen caging combined with oxalic acid treatment to control varroa mites (Varroa destructor) in honey bee colonies (Apis mellifera). Apidologie. https://doi.org/10.1007/s13592-017-0526-2.
Imdorf, A., Buehlmann, G., Gerig, L., Kilckenmann, V., Wille, H. (1987) Überprüfung der Schätzmethode zur Ermittlung der Brutfläche und der Anzahl Arbeiterinnen in freifliegenden Bienenvölkern. Apidologie 18(2), 137–146. https://doi.org/10.1051/apido:19870204.
Jaffé R., V. Dietemann, M.H. Allsopp, C. Costa, R.M. Crewe, et al. (2010) Estimating the density of honeybee colonies across their natural range to fill the gap in pollinator decline consensus. Conserv. Biol. 24, 583–593.
Lee, K.V., Steinhauer, N., Rennich, K., Wilson, M.E., Tarpy, D.R., Caron, D.M. (2015) A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie 46, 292–305.
Lodesani, M., Costa, C. (2005) Limits of chemotherapy in beekeeping: development of resistance and the problem of residues. Bee World 86(4), 102–109.
Lodesani, M., Colombo, M., Spreafico, M. (1995) Ineffectiveness of Apistan treatment against the mite Varroa jacobsoni Oud. in several districts of Lombardy (Italy). Apidologie 26, 67–72.
Lodesani, M., Crailsheim, K., Moritz, R.F.A., (2002) Effect of some characters on the population growth of mite Varroa jacobsoni in Apis mellifera L colonies and results of a bi-directional selection. J. Appl. Entomol. 126(2–3), 130–137.
Lodesani M., Costa C., Besana A., Dall’Olio R., Franceschetti S., Tesoriero D., Vaccari G., (2014) Impact of control strategies for Varroa destructor on colony survival and health in northern and central regions of Italy. J. Apic. Res. 53(1), 155–164. https://doi.org/10.3896/IBRA.1.53.1.17
Maini, S., Medrzycki, P., Porrini, C. (2010) The puzzle of honey bee losses: a brief review. B. Insectol. 63, 153–160.
Martin, S. J. (2001) The role of Varroa and viral pathogens in the collapse of honeybee colonies: a modelling approach. J. Appl. Ecol. 38, 1082.
Martin, S.J., Highfield, A.H., Brettell, L., Villalobos, E.M., Budge, G.E., Powell, M., Nikaido, S., Schroeder, D.C. (2012) Global honey bee viral landscape altered by a parasitic mite. Science 336, 6086, 1304–1306. https://doi.org/10.1126/science.1220941
Maul, V., Klepsch, A., Assmannwerthmuller, U. (1988) The trapping comb technique as part of bee management under strong infestation by Varroa jacobsoni Oud. Apidologie 19, 139–154.
Metcalf, R. L, (1982) Insecticides in pest management. In Metcalf, R L; Luckmann, W H (eds) Introduction to insect pest management. Wiley; New York, pp. 217–277 (2nd edition).
Mutinelli, F., Costa, C., Lodesani, M., Baggio, A., Medrzycki, P., Formato, G., et al. (2010 Honey bee colony losses in Italy. J. Apic. Res. 49, 119–120.
Nanetti, A., Besana, A.M., Baracani, G., Romanelli, R., Galuppi, R. (2011) Artificial brood interruption in combination with oxalic acid trickling in the control of varroa mite. In Proceedings of 42nd International Apicultural Congress, Buenos Aires, Argentina.
Nanetti, A., Higes, M., Baracani, G., Besana, A. (2012) Control de la varroa con ácido oxálico en combinación con la interrupción artificial de la puesta de cría. Proceeding of II Congreso Ibérico de Apicultura, Guadalajara, Spain, October 18–20.
Nanetti, A., Büchler, R., Uzunov, A., Gregorc, A. (2016) Coloss - varroa control taskforce, brood interruption study 2016/2017. http://www.coloss.org/taskforces/varroacontrol/protocols-brood-interruption- varroa-task-coloss-2016-jan-final
Nazzi, F., Le Conte, Y. (2016) Ecology of Varroa destructor, the major ectoparasite of the Western Honey Bee, Apis mellifera. Annu. Rev. Entomol. 61, 417–432.
Pietropaoli, M., Giacomelli, A., Milito, M., Gobbi, C., Scholl, F., Formato, G. (2012) Integrated pest management strategies against Varroa destructor, the use of oxalic acid combined with innovative cages to obtain the absence of brood. Eur. J. Integr. Med. 15, 93.
Ramsey, S. D., van Engelsdorp, D. (2016) Varroa destructor feed primarily on honey bee fat body not hemolymph. Bee World 93, 117
Ravoet, J., De Smet, L., Wenseleers, T., de Graaf, DC. (2015) Genome sequence heterogeneity of Lake Sinai Virus found in honey bees and Orf1/RdRP-based polymorphisms in a single host. Virus Res. 201, 67–72. https://doi.org/10.1016/j.virusres.2015.02.019.
Rosenkranz, P., Aumeier, P., Ziegelmann, B. (2010) Biology and control of Varroa destructor. J. Invertebr. Pathol. 103, S96–S119. https://doi.org/10.1016/j.681jip.2009.07.016
Statsoft, Inc. (2008) STATISTICA (data analysis software system), version 7.1. www.statsoft.com
Underwood, R.M., van Engelsdorp, D. (2007) Colony collapse disorder: have we seen this before? Bee Culture 135, 13–18.
VanEngelsdorp, D., Evans, J.D., Saegerman, C., Mullin, C., Haubruge, E., Nguyen, B.K., et al. (2009) Colony collapse disorder: a descriptive study. PLoS One 4, e6481. https://doi.org/10.1371/journal.pone.0006481
Acknowledgments
We wish to thank Cecilia Costa for her corrections and suggestions that improved the readability of the manuscript.
Funding
This work was supported by Italian Ministry of Agriculture, Food and Forestry through EU Reg. 1308/2017–2019.
Author information
Authors and Affiliations
Contributions
SF performed field test and colony management; RD performed the experiments and analysis and contributed to writing the paper. All authors read and approved the final manuscript.
Corresponding author
Additional information
Manuscript editor: Yves Le Conte
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Évaluation des méthodes de gestion biotechnique au début du printemps pour lutter contre la varroatose chez Apis mellifera
Contrôle de Varroa / Varroa destructor / rayon-piège / cage de reine / bio-techniques
Beurteilung von biotechnischen Verfahren zum Frühlingsbeginn als Alternativen zur Varroa-Kontrolle bei Apis mellifera.
Varroa-Kontrolle /Varroa destructor / Wabenfallen / Käfigung von Königinnen / Biotechnische Verfahren
Rights and permissions
About this article
Cite this article
Lodesani, M., Franceschetti, S. & Dall’Ollio, R. Evaluation of early spring bio-technical management techniques to control varroosis in Apis mellifera. Apidologie 50, 131–140 (2019). https://doi.org/10.1007/s13592-018-0621-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-018-0621-z