Abstract
Purpose
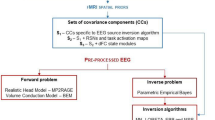
Although fMRI constrained EEG/MEG source imaging can enhance spatiotemporal resolutions of functional neuroimaging, it has been reported that hard fMRI constraint can result in misidentification of neuronal sources if severe mismatches exist between fMRI activations and EEG/MEG sources. In our previous works, we proposed an approach to address this issue, which automatically adjusts the strength of fMRI constraints by considering the mismatch level. The previous studies proved to be useful particularly when one wants to obtain actual EEG/MEG source locations and uses fMRI prior information as an auxiliary tool to enhance focality of the distributed sources. However, some loss of concentration in the reconstructed images was inevitable when distinct mismatches exist
Methods
In this study, instead of automatically extending the prior activation regions, we classified and labeled distinct prior activation regions, compared each of the fMRI prior activation regions with each of the thresholded EEG/MEG activation regions, excluded the matched EEG/MEG activation regions, and produced modified prior activation regions having smaller areas than the extended prior activation regions obtained using the conventional approach.
Results
A series of realistic EEG simulations, assuming fMRI invisible and discrepancy sources, showed that the improved approach not only compensated for the distortions due to the mismatched activations, but also maintained the high spatial resolution of the fMRI constrained EEG/MEG source imaging.
Conclusions
Our simulation results demonstrated that the proposed technique can be a promising option to deal with mismatches between fMRI and EEG/MEG sources in the fMRI constrained EEG/MEG source imaging.
Similar content being viewed by others
References
Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000; 26:55–67.
Ahlfors SP, Simpson GV, Dale AM, Belliveau JW, Liu AK, Korvenoja A, Virtanen J, Huotilainen M, Tootell RBH, Aronen HJ, Ilmoniemi RJ. Spatiotemporal activity of a cortical network for processing visual motion revealed by MEG and fMRI. J Neurophysiol. 1999; 82:2545–2555.
Korvenoja A, Huttunen J, Salli E, Pohjonen H, Martinkauppi S, Palva JM, Lauronen L, Virtanen J, Ilmoniemi RJ, Aronen HJ. Activation of multiple cortical areas in response to somatosensory stimulation: combined magnetoencephalographic and functional magnetic resonance imaging. Hum Brain Mapp. 1999; 8:13–27.
Korvenoja A, Aronen HJ, Ilmoniemi J. Functional MRI as a constraint in multi-dipole models of MEG data. Int J Bioelectrom. 2001; 3 (internet journal).
Wagner M, Fuchs M, Kastner J. fMRI-constrained dipole fits and current density reconstructions. Proc Biomag2000. 2000.
Dale AM, Sereno M I. Improved localization of cortical activity by combining EEG and MEG with MRI surface reconstruction: a linear approach. J. Cognit. Neurosci. 1993; 5:162–176.
George JS, Aine CJ, Mosher JC, Schmidt DM, Ranken DM, Schlitt HA, Wood CC, Lewine JD, Sanders JA, Belliveau JW. Mapping function in the human brain with magnetoencephlaography, anatomical magnetic-resonance-imaging, and functional magnetic-resonance-imaging. J Clin Neurophysiol. 1995; 12:406–431.
Phillips C, Mattout J, Rugg MD, Maquet P, Friston KJ. An empirical Baysian solution to the source reconstruction problem in EEG. Neuroimage. 2005; 24:997–1011.
Liu AK, Belliveau JW, Dale AM. Spatiotemporal imaging of human brain activity using functional MRI constrained magnetoencephalography data: Monte Carlo simulations. Proc Natl Acad Sci USA. 1998; 95:8945–8950.
Babiloni F, Babiloni C, Carducci F, Romani GL, Rossini PM, Angelone LM, Cincotti F. Multimodal integration of high-resolution EEG and functional magnetic resonance imaging data: a simulation study. Neuroimage. 2003; 19:1–15.
Opitz B, Mecklinger A, von Cramon DY, Kruggel F. Combining electrophysiological and hemodynamic measures of the auditory oddball. Phychophysiol. 1999; 36:142–147.
Torquati K, Pizzella V, Babiloni C, Gratta CD, Penna SD, Ferretti A, Franciotti R, Rossini PM, Romani GL. Nociceptive and non-nociceptive sub-regions in the human secondary somatosensory cortex: an MEG study using fMRI constraints. Neuroimage. 2005; 26:48–56.
Vanni S, Warnking J, Dojat M, Delon-Martin C, Bullier J, Segebarth C. Sequence of pattern onset responses in the human visual areas: an fMRI constrained VEP source analysis. Neuroimage. 2004; 21:801–817.
Liu AK, Dale AM, Belliveau JW. Monte Carlo simulation studies of EEG and MEG localization accuracy. Hum Brain Mapp. 2002; 16:47–62.
Bonmassar G, Schwartz DP, Liu AK, Kwong KK, Dale AM, Belliveau JW. Spatiotemporal brain imaging of visual-evoked activity using interleaved EEG and fMRI recordings. Neuroimage. 2001; 13:1035–1043.
Lin FH, Witzel T, Hämäläinen MS, Dale AM, Belliveau JW, Stufflebeam SM. Spectral spatiotemporal imaging of cortical oscillations and interactions in the human brain. Neuroimage. 2004; 23:582–595.
Vitacco D, Brandeis D, Pascual-Marqui R, Martin E. Correspondence of event-related potential tomography and functional magnetic resonance imaging during language processing. Hum Brain Mapp. 2002; 17:4–12.
Gonzalez Andino SL, Blanke O, Lantz G, Thut G, Grave de Peralta Menendez R. The use of functional constraints for the neuroelectromagnetic inverse problem: alternatives and caveats. Int J Bioelectrom. 2001; 3 (internet journal).
Ahlfors SP, Simpson GV. Geometrical interpretation of fMRIguided MEG/EEG inverse estimates. Neuroimage. 2004; 22:323–332.
Im CH, Lee SY. A Technique to consider mismatches between fMRI and EEG/MEG sources for fMRI constrained EEG/MEG source imaging: a preliminary simulation study. Phys Med Biol. 2006; 51:6005–6021.
Fujimaki N, Hayakawa T, Nielsen M, Knösche TR, Miyauchi S. An fMRI-constrained MEG source analysis with procedures for dividing and grouping activation. Neuroimage. 2002; 17:324–343.
Im CH, Jung HK, Fujimaki N. fMRI-constrained MEG source imaging and consideration of fMRI invisible sources. Hum Brain Mapp. 2005; 26:110–118.
Liu Z, Ding L, He B. Integration of EEG/MEG with MRI and fMRI in functional neuroimaging. IEEE Eng Med Biol Mag. 2006; 25:46–53.
Im CH. Dealing with mismatched fMRI activations in fMRI constrained EEG cortical source imaging: a simulation study assuming various mismatch types. Med Biol Eng Comput. 2007; 45:79–90.
He B, Musha T, Okamoto Y, Homma S, Nakajima Y, Sato T. Electric dipole tracing in the brain by means of the boundary element method and its accuracy. IEEE Trans Biomed Eng. 1987; 34:406–414.
Hämäläinen MS, Sarvas J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng. 1989; 36:165–171.
Haueisen J, Ramon C, Eiselt M, Brauer H, Nowak H. Influence of tissue resistivities on neuromagnetic fields and electric potentials studied with a finite element model of the head. IEEE Trans Biomed Eng. 1997; 44:727–735.
Oostendorp TF, Delbeke J, Stegeman DF. The conductivity of the human skull: results of in vivo and in vitro measurements. IEEE Trans Biomed Eng. 2000; 47:1487–1492.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis I. Segmentation and surface reconstruction. Neuroimage. 1999; 9:179–194.
Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000; 97:11050–11055.
Dhond RP, Marinkovic K, Dale AM, Witzel T, Halgren E. Spatiotemporal maps of past-tense verb inflection. Neuroimage. 2003; 19:91–100.
Gorodnistky IF, George JS, Rao BD. Neuromagnetic imaging with FOCUSS: a recursive weighted minimum norm algorithm. Electroenceph Clin Neurophys. 1995; 95:231–251.
Hansen P. Analysis of discrete ill-posed problems by means of the L-curve. SIAM Rev. 1992; 34:561–580.
Höppner F, Klawonn F, Kruse R, Runkler T. Fuzzy cluster analysis: methods for classification, data analysis and image recognition. New York: John Wiley & Sons; 1999.
Bedzek JC, Pal NR. Some new indexes for cluster validity. IEEE Trans Systems Man Cybernet-Part-B. 1998; 28:301–315.
Kincses WE, Braun C, Kaiser S, Elbert T. Modeling extended sources of event-related potentials using anatomical and physiological constraints. Hum Brain Mapp. 1999; 8:182–193.
Sekihara K, Nagarajan SS, Poeppel D, Marantz A, Miyashita Y. Reconstructing spatio-temporal activities of neural sources using an MEG vector beamformer technique. IEEE Trans Biomed Eng. 2001; 48:760–771.
Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002; 6:129–142.
Liu ZM, He B. fMRI-EEG integrated cortical source imaging by use of time-variant spatial constraints. Neuroimage. 2008; 39:1198–1214.
Ou W, Nummenmaa A, Ahveninen J, Belliveau JW, Hämäläinen MS, Golland P. Multimodal functional imaging using fMRIinformed regional EEG/MEG source estimation. Neuroimage. 2010; 52:79–108.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, YJ., Im, CH. An improved technique to consider mismatches between fMRI and EEG/MEG sources for fMRI constrained EEG/MEG source imaging. Biomed. Eng. Lett. 1, 32–41 (2011). https://doi.org/10.1007/s13534-011-0002-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-011-0002-2