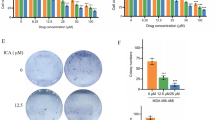
Abstract
Purpose
Previously, we reported that quinacrine (QC) may cause apoptosis in breast and colon cancer cells by activating the death receptor 5 (DR5), resulting in autophagic cell death through p21 modulation. Here, we systematically evaluated the combined role of p21 and DR5 and their crosstalk in QC-mediated autophagy and apoptosis in breast cancer cells using in vitro and in vivo models.
Methods
Multiple breast cancer-derived cell lines (MCF-7, ZR-75-1, T47D, MDA-MB-231 and MCF-10A-Tr) and a mouse xenograft model were used. Also, multiple assays, including Western blotting, immunoprecipitation, staining for autophagy and apoptosis, gene silencing, hematoxylin and eosin staining, immunohistochemistry, cell viability assessment, fluorescence imaging and cell sorting were used.
Results
We found that QC activates p21 and DR5 in combination with the apoptosis inducer TRAIL in the breast cancer-derived cells tested. Combined TRAIL and QC treatment increased autophagy and apoptosis by increasing the interaction between, and co-localization of, p21 and DR5 in the death-inducing signaling complex (DISC). We found that this combination also inhibited the mTOR/PI3K/AKT signaling cascade and modulated reactive oxygen species (ROS) and nitric oxide (NO) production. Reductions in autophagy and apoptosis in DR5-knockout cells and a lack of change in p21-DR5-silenced cells were noted after TRAIL + QC treatment. This result explains dependence of the death (autophagy and apoptosis) cascade on these two key regulatory proteins. In addition, we found in an in vivo mouse xenograft model that increased expression and enhanced co-localization of p21 and DR5 after TRAIL + QC treatment supported a joint regulatory role of these proteins in the co-prevalence of autophagy and apoptosis.
Conclusion
Our data suggest that a combined treatment of TRAIL and QC causes cell death in breast cancer-derived cells via autophagy and apoptosis by increasing the interaction of p21 and DR5, as indicated by both in vitro and in vivo studies.








Similar content being viewed by others
References
L. Lin, E.H. Baehrecke, Autophagy, cell death, and cancer. Mol. Cell. Oncol. 2, e985913 (2015)
T. Yonekawa, A. Thorburn, Autophagy and cell death. Essays Biochem. 55, 105–117 (2014)
A. Thorburn, Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis 13, 1–9 (2008)
P.M. Yang, C.C. Chen, Life or death? Autophagy in anticancer therapies with statins and histone deacetylase inhibitors. Autophagy 7, 107–108 (2011)
T.W. Poh, S. Huang, J.L. Hirpara, S. Pervaiz, LY303511 amplifies TRAIL-induced apoptosis in tumor cells by enhancing DR5 oligomerization, DISC assembly, and mitochondrial permeabilization. Cell Death Differ. 14, 1813–1825 (2007)
X. Li, M. You, Y.J. Liu, L. Ma, P.P. Jin, R. Zhou, Z.X. Zhang, B. Hua, X.J. Ji, X.Y. Cheng, F. Yin, Y. Chen, W. Yin, Reversal of the apoptotic resistance of non-small cell lung carcinoma towards TRAIL by natural product Toosendanin. Sci. Rep. 7, 42748 (2017)
S. Das, N. Tripathi, R. Preet, S. Siddharth, A. Nayak, P.V. Bharatam, C.N. Kundu, Quinacrine induces apoptosis in cancer cells by forming a functional bridge between TRAIL-DR5 complex and modulating the mitochondrial intrinsic cascade. Oncotarget 8, 248–267 (2017)
J.D. Twomey, S.R. Kim, L. Zhao, W.P. Bozza, B. Zhang, Spatial dynamics of TRAIL death receptors in cancer cells. Drug Resist. Updat. 19, 13–21 (2015)
J. Han, W. Hou, L.A. Goldstein, C. Lu, D.B. Stolz, X.M. Yin, H. Rabinowich, Involvement of protective autophagy in TRAIL resistance of apoptosis-defective tumor cells. J. Biol. Chem. 283, 19665–19677 (2008)
X. Di, G. Zhang, Y. Zhang, K. Takeda, L.A. Rivera Rosado, B. Zhang, Accumulation of autophagosomes in breast cancer cells induces TRAIL resistance through downregulation of surface expression of death receptors 4 and 5. Oncotarget 4, 1349–1364 (2013)
W. Ouyang, C. Yang, Y. Liu, J. Xiong, J. Zhang, Y. Zhong, G. Zhang, F. Zhou, Y. Zhou, C. Xie, Redistribution of DR4 and DR5 in lipid rafts accounts for the sensitivity to TRAIL in NSCLC cells. Int. J. Oncol. 39, 1577–1586 (2011)
L. Xu, X. Hu, X. Qu, K. Hou, H. Zheng, Y. Liu, Cetuximab enhances TRAIL induced gastric cancer cell apoptosis by promoting DISC formation in lipid rafts. Biochem. Biophys. Res. Commun. 439, 285–290 (2013)
D. Wolczyk, M. Zaremba-Czogalla, A. Hryniewicz-Jankowska, R. Tabola, K. Grabowski, A.F. Sikorski, K. Augoff, TNF-α promotes breast cancer cell migration and enhances the concentration of membrane-associated proteases in lipid rafts. Cell. Oncol. 39, 353–363 (2016)
W. He, Q. Wang, J. Xu, X. Xu, M.T. Padilla, G. Ren, X. Gou, Y. Lin, Attenuation of TNFSF10/TRAIL-induced apoptosis by an autophagic survival pathway involving T. Autophagy 8, 1811–1821 (2012)
W. Hou, J. Han, C. Lu, L.A. Goldstein, H. Rabinowich, Autophagic degradation of active caspase-8: A crosstalk mechanism between autophagy and apoptosis. Autophagy 6, 891–900 (2010)
G. Herrero-Martin, M. Hoyer-Hansen, C. Garcia-Garcia, C. Fumarola, T. Farkas, A. Lopez-Rivas, M. Jaattela, TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 28, 677–685 (2009)
X. Li, X. Li, J. Wang, Z. Ye, J.C. Li, Oridonin up-regulates expression of p21 and induces autophagy and apoptosis in human prostate cancer cells. Int. J. Biol. Sci. 8, 901–912 (2012)
S. Liu, W. Lu, S. Li, S. Li, J. Liu, Y. Xing, S. Zhang, J. Zhongxiang Zhou, H. Xing, Y. Xu, Q. Rao, C. Deng, M. Wang, J. Wang, Identification of JL1037 as a novel, specific, reversible lysine-specific demethylase 1 inhibitor that induce apoptosis and autophagy of AML cells. Oncotarget 8, 31901–31914 (2017)
A. Nagappan, W.S. Lee, J.W. Yun, J.N. Lu, S.H. Chang, J.H. Jeong, G.S. Kim, J.M. Jung, S.C. Hong, Tetraarsenic hexoxide induces G2/M arrest, apoptosis, and autophagy via PI3K/Akt suppression and p38 MAPK activation in SW620 human colon cancer cells. PLoS One 12, e0174591 (2017)
J.L. Chang, J.M. Chow, J.H. Chang, Y.C. Wen, Y.W. Lin, S.F. Yang, W.J. Lee, M.H. Chien, Quercetin simultaneously induces G(0) /G(1) phase arrest and caspase-mediated crosstalk between apoptosis and autophagy in human leukemia HL-60 cells. Environ. Toxicol. 32, 1857–1868 (2017)
R. Preet, P. Mohapatra, S. Mohanty, S.K. Sahu, T. Choudhuri, M.D. Wyatt, C.N. Kundu, Quinacrine has anticancer activity in breast cancer cells through inhibition of topoisomerase activity. Int. J. Cancer 130, 1660–1670 (2012)
R. Preet, P. Mohapatra, D. Das, S.R. Satapathy, T. Choudhuri, M.D. Wyatt, C.N. Kundu, Lycopene synergistically enhances quinacrine action to inhibit Wnt-TCF signaling in breast cancer cells through APC. Carcinogenesis 34, 277–286 (2013)
R. Preet, S. Siddharth, S.R. Satapathy, S. Das, A. Nayak, D. Das, M.D. Wyatt, C.N. Kundu, Chk1 inhibitor synergizes quinacrine mediated apoptosis in breast cancer cells by compromising the base excision repair cascade. Biochem. Pharmacol. 105, 23–33 (2016)
J. Ge, Y. Liu, Q. Li, X. Guo, L. Gu, Z.G. Ma, Y.P. Zhu, Resveratrol induces apoptosis and autophagy in T-cell acute lymphoblastic leukemia cells by inhibiting Akt/mTOR and activating p38-MAPK. Biomed. Environ. Sci. 26, 902–911 (2013)
P. Mohapatra, R. Preet, D. Das, S.R. Satapathy, T. Choudhuri, M.D. Wyatt, C.N. Kundu, Quinacrine-mediated autophagy and apoptosis in colon cancer cells is through a p53- and p21-dependent mechanism. Oncol. Res. 20, 81–91 (2012)
P. Mohapatra, R. Preet, D. Das, S.R. Satapathy, S. Siddharth, T. Choudhuri, M.D. Wyatt, C.N. Kundu, The contribution of heavy metals in cigarette smoke condensate to malignant transformation of breast epithelial cells and in vivo initiation of neoplasia through induction of a PI3K-AKT-NFκB cascade. Toxicol. Appl. Pharmacol. 1, 168–179 (2014)
S.R. Satapathy, P. Mohapatra, D. Das, S. Siddharth, C.N. Kundu, The apoptotic effect of plant based Nanosilver in colon cancer cells is a p53 dependent process involving ROS and JNK cascade. Pathol. Oncol. Res. 21, 405–411 (2015)
D. Das, S.R. Satapathy, S. Siddharth, A. Nayak, C.N. Kundu, NECTIN-4 increased the 5-FU resistance in colon cancer cells by inducing the PI3K-AKT cascade. Cancer Chemother. Pharmacol. 76, 471–479 (2015)
S.S. Mpoke, J. Wolfe, Differential staining of apoptotic nuclei in living cells: Application to macronuclear elimination in Tetrahymena. J. Histochem. Cytochem. 45, 675–683 (1997)
T.S. Jani, J. DeVecchio, T. Mazumdar, A. Agyeman, J.A. Houghton, Inhibition of NF-kappaB signaling by quinacrine is cytotoxic to human colon carcinoma cell lines and is synergistic in combination with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or oxaliplatin. J. Biol. Chem. 285, 19162–19172 (2010)
W. Wang, J.N. Gallant, S.I. Katz, N.G. Dolloff, C.D. Smith, J. Abdulghani, J.E. Allen, D.T. Dicker, B. Hong, A. Navaraj, W.S. El-Deiry, Quinacrine sensitizes hepatocellular carcinoma cells to TRAIL and chemotherapeutic agents. Cancer Biol. Ther. 12, 229–238 (2011)
H. Liu, L. Zhang, X. Zhang, Z. Cui, PI3K/AKT/mTOR pathway promotes progestin resistance in endometrial cancer cells by inhibition of autophagy. Onco. Targets. Ther. 10, 2865–2871 (2017)
R. Ehsanian, C.V. Waes, S.M. Feller, Beyond DNA binding - a review of the potential mechanisms mediating quinacrine’s therapeutic activities in parasitic infections, inflammation, and cancers. Cell Commun. Signal 15, 9–13 (2011)
C. Guo, A.V. Gasparian, Z. Zhuang, D.A. Bosykh, A.A. Komar, A.V. Gudkov, K.V. Gurova, 9-Aminoacridine-based anticancer drugs target the PI3K/AKT/mTOR, NF-kappaB and p53 pathways. Oncogene 26, 1151–1161 (2009)
M.R. Lobo, X. Wang, Y. Gillespie, R.L. Woltjer, M.M. Pike, Combined efficacy of Cediranib and Quinacrine in glioma is enhanced by hypoxia and causally linked to autophagic vacuole accumulation. PLoS One 9, e114110 (2014)
D. Marklein, U. Graab, I. Naumann, T. Yan, R. Ridzewski, F. Nitzki, A. Rosenberger, K. Dittmann, J. Wienands, L. Wojnowski, S. Fulda, H. Hahn, PI3K Inhibition enhances doxorubicin-induced apoptosis in sarcoma cells. PLoS One 7, e52898 (2012)
Y. Tong, W. Zhu, X. Huang, L. You, X. Han, C. Yang, W. Qian, PI3K Inhibitor LY294002 inhibits activation of the Akt/mTOR pathway induced by an oncolytic adenovirus expressing TRAIL and sensitizes multiple myeloma cells to the oncolytic virus. Oncol. Rep. 31, 1581–1588 (2014)
Y. Cai, X. Tan, J. Liu, Y. Shen, D. Wu, M. Ren, P. Huang, D. Yu, Inhibition of PI3K/Akt/mTOR signaling pathway enhances the sensitivity of the SKOV3/DDP ovarian cancer cell line to cisplatin in vitro. Chin. J. Cancer Res. 26, 564–572 (2014)
Acknowledgements
We sincerely acknowledge the Department of Science and Technology (DST) and the Indian Council of Medical Research (ICMR), Government of India, for providing fellowships to SD, AN and SS, respectively. In addition, we sincerely thank Mark Zakshevsky, Department of Anatomy and Cell Biology, University of Florida, Gainesville, Florida, USA, for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 710 kb)
Rights and permissions
About this article
Cite this article
Das, S., Nayak, A., Siddharth, S. et al. TRAIL enhances quinacrine-mediated apoptosis in breast cancer cells through induction of autophagy via modulation of p21 and DR5 interactions. Cell Oncol. 40, 593–607 (2017). https://doi.org/10.1007/s13402-017-0347-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-017-0347-3