Abstract
Background
Chemotherapy-induced immune suppression has mainly been studied in patients with advanced cancer, but the influence of chemotherapy on the immune system in early stage cancer patients has so far not been studied systematically. The aim of the present study was to monitor the immune system during anthracycline- and taxane-based adjuvant chemotherapy in early stage breast cancer patients, to assess the impact of circulating tumor cells on selected immune parameters and to reveal putative angiogenic effects of circulating endothelial cells.
Methods
Peripheral blood samples from 20 early stage breast cancer patients were analyzed using a flow cytometric multi-color of antibodies to enumerate lymphocyte and dendritic cell subsets, as well as endothelial and tumor cells. An enzyme-linked immunosorbent assay (ELISA) was used to measure the levels of various serological factors.
Results
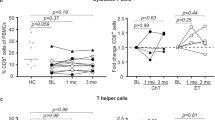
During chemotherapy, all immunological parameters and angiogenesis surrogate biomarkers showed significant decreases. The numbers of circulating tumor cells showed significant inverse correlations with the numbers of T helper cells, a lymphocyte subset directly related to effective anti-tumor responses. Reduced T helper cell numbers may contribute to systemic immunosuppression and, as such, the activation of dormant tumor cells.
Conclusions
From our results we conclude that adjuvant chemotherapy suppresses immune function in early stage breast cancer patients. In addition, we conclude that the presence of circulating tumor cells, defined as pan-cytokeratin+, CD326+, CD45− cells, may serve as an important indicator of a patient’s immune status. Further investigations are needed to firmly define circulating tumor cells as a predictor for the success of breast cancer adjuvant chemotherapy.






Similar content being viewed by others
References
R.V. Gardner, Long term hematopoietic damage after chemotherapy and cytokine. Front. Biosci. 4, e47–e57 (1999)
R.G. Van der Most, A. Currie, B.W. Robinson, R.A. Lake, Cranking the immunologic engine with chemotherapy: using context to drive tumor antigen cross-presentation towards useful antitumor immunity. Cancer Res. 66, 601–604 (2006)
D.H. Kang, M.T. Weaver, N.J. Park, B. Smith, T. McArdle, J. Carpenter, Significant impairment in immune recovery after cancer treatment. Nurs. Res. 58, 105–114 (2009)
M.J. Campbell, J. Scott, H.T. Maecker, J.W. Park, L.J. Esserman, Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Res. Treat. 91, 163–171 (2005)
I. Caras, A. Grigorescu, C. Stavaru, D.L. Radu, I. Mogos, G. Szegli, A. Salageanu, Evidence for immune defects in breast and lung cancer patients. Cancer Immunol. Immunother. 53, 1146–1152 (2004)
G. Konievic, I. Spuzic, Stage dependence of NK cell activity and its modulation by interleukin 2 in patients with breast cancer. Neoplasma 40, 81–855 (1993)
C. Wiltschke, M. Krainer, A.C. Budinsky, A. Berger, C. Muller, R. Zeillinger, P. Speiser, E. Kubista, M. Eibl, C.C. Zeilinski, Reduced mitogenic stimulation of peripheral blood mononuclear cells as a prognostic parameter for the course of breast cancer: a prospective longitudinal study. Br. J. Cancer 71, 1292–1296 (1995)
Y. Nieto, E.J. Shpall, I.K. McNiece, S. Nawaz, J. Beaudet, S. Rosinski, J. Pellom, V. Slat-Vasquez, P.A. McSweeney, S.I. Bearman, J. Murphy, R.B. Jones, Prognostic analysis of early lymphopcyte recovery in patients with advanced breast cancer receiving high-dose chemotherapy with an autologous hematopoietic progenitor cell transplant. Clin. Cancer Res. 10, 5076–5086 (2004)
I.F. Porrata, J.N. Ingle, M.R. Litzow, S. Geyer, S.N. Markovic, Prolonged survival associated wit early lymphocyte recovery after autologous hematopoietic stem cell transplantation for patients with metastatic breast cancer. Bone Marrow Transplant. 28, 865–871 (2001)
S. Mariucci, B. Rovati, M. Manzoni, M.G. Della Porta, G. Comolli, S. Delfanti, M. Danova, Lymphocyte subpopulation and dendritic cell phenotyping during antineoplastic therapy in human solid tumors. Clin. Exp. Med. 11, 199–210 (2011)
G. Ferlazzo, M. Pack, D. Thomas, C. Paludan, D. Schmid, T. Strowing, G. Bougras, V.A. Muller, L. Moretta, C. Munz, Distinct CT roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc. Natl. Acad. Sci. U. S. A. 101, 16606–16611 (2004)
S. Zhao, Y. Liu, Q. Zhang, H. Li, M. Zhang, W. Ma, W. Zhao, J. Wang, M. Yang, The prognostic role of circulating tumor cells (CTCs) detected by RT-PCR in breast cancer. A meta-analysis of published literature. Breast Cancer Res. Treat. 130, 809–816 (2011)
L.D. Wood, D.W. Parsons, S. Jones, J. Lin, T. Sjoblom, R.J. Leary, D. Shen, S.M. Boca, T. Barber, J. Ptak, N. Silliman, S. Szabo, Z. Dezso, V. Ustyanksky, T. Nikolskaya, Y. Nikolsky, R. Karchin, P.A. Wilson, J.S. Kaminker, Z. Zhang, R. Croshaw, J. Willis, D. Dawson, M. Shipitsin, J.K. Willson, S. Sukumar, K. Polyak, B.H. Park, C.L. Pethiyagoda, P.V. Pant, D.G. Ballinger, A.B. Sparks, J. Hartigan, D.R. Smith, E. Suh, N. Papadopoulos, P. Buckhaults, S.D. Markowitz, G. Parmigiani, K.W. Kinzler, V.E. Velculescu, B. Vogelstein, The genomic landscapes of human breast and colorectal cancers. Science 318, 1108–1113 (2007)
C.A. Klein, D. Holzel, Systemic cancer progression and tumor dormancy: mathematical models meet single cell genomics. Cell Cycle 5, 1788–1798 (2006)
S. North, M. Moenner, A. Bikfalvi, Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Lett. 218, 1–14 (2005)
M. Relf, S. LeJeune, P.A. Scott, S. Fox, K. Smith, R. Leek, A. Moghaddam, R. Whitehouse, R. Bicknell, A.L. Harris, Expression of the angiogenic factors vascular endothelial cells growth factors, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 57, 963–969 (1997)
M. Campoli, S. Ferrone, A.H. Zea, P.C. Rodriguez, A.C. Ochoa, Mechanisms of tumor evasion. Cancer Treat. Res. 23, 61–88 (2005)
H.R. Salih, V. Nüssler, Immune escape versus tumor tolerance: how do tumor evade immune surveillance? Eur. J. Med. Res. 6, 323–632 (2001)
C.C. Lin, C.Y. Liu, M.J. Chen, T.E. Wang, C.H. Chu, H.Y. Wang, S.C. Shit, M.L. Hsu, T.C. Hsu, Y.J. Chen, Profiles of circulating endothelial cells and serum cytokines during adjuvant chemoradiation in rectal cancer patients. Clin. Transl. Oncol. 15, 855–860 (2013)
E. Razis, K.T. Kalogeras, V. Kotoula, A.G. Eleftheraki, N. Nikitas, R. Kronenwett, E. Timotheadou, C. Christodoulou, D. Pectasides, H. Gogas, R.M. Wirtz, T. Makatsoris, D. Bafaloukos, G. Aravantinos, D. Televantou, N. Pavlidis, G. Fountzilas, Improved outcome of high-risk early HER2 positive breast cancer with high CXCL13-CXCR5 messenger RNA expression. Clin. Breast Cancer 12, 183–193 (2012)
J. Wang, Q. He, Y.G. Shao, M. Ji, Chemokine fluctuate in the progression of primary breast cancer. Eur. Rev. Med. Farmacol. Sci. 17, 596–608 (2013)
World Medical Association, Declaration of Helsinki: ethical principles for medical research involving human subjects. J. Postgrad. Med. 48, 206–208 (2002)
B. Rovati, S. Mariucci, R. Poma, C. Tinelli, S. Delfanti, P. Pedrazzoli, An eight-colour flow cytometric method for the detection of reference values of lymphocyte subsets in selected healthy donors. Clin. Exp. Med. 14, 249–259 (2014)
B. Rovati, S. Mariucci, M. Manzoni, K. Bencardino, M. Danova, Flow cytometric detection of circulating dendritics cells in healthy subjects. EJH 52, 45–52 (2008)
S. Mariucci, B. Rovati, S. Chatzileontiadou, K. Bencardino, M. Manzoni, S. Delfanti, M. Danova, A six-colour flow cytometric method for simultaneous detection of cell phenotype and apoptosis of circulating endothelial cells. Scand. J. Clin. Lab. Invest. 69, 433–438 (2009)
L. Whitby, W. Granger, I. Storie, K. Goodfellow, A. Sawle, J.T. Reilly, D. Barnett, Quality control of CD4+ T-lymphocyte enumeration : results from 9 years of the United Kingdom national external quality assessment scheme for immune monitoring (1993–2001). Cytometry 50, 102–110 (2002)
T. Hristozova, R. Konschak, C. Stromberger, A. Fusi, Z. Liu, W. Weichert, A. Stenzinger, V. Budach, U. Keilholz, I. Tinhofer, The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN). Ann. Oncol. 22, 1878–1885 (2011)
T.J. Molloy, A.J. Bosma, L.O. Baumbusch, M. Synnestvedt, E. Borgen, H.G. Russnes, E. Schlichting, L.J. van’t Veer, B. Naume, The prognostic significance of tumor cell detection in the peripheral blood versus the bone marrow in 733 early-stage breast cancer patients. Breast Cancer Res. 3, R61 (2011)
C. Jochems, J. Schlom, Tumor- infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp. Biol. Med. 236, 567–279 (2011)
I. Poschke, D. Mougiakakos, R. Kiessling, Camouflage and sabotage: tumor escape from the immune system. Cancer Immunol. Immunother. 60, 1161–1171 (2011)
J. de Boniface, I. Poschke, Y. Mao, R. Kiessling, Tumor-dependent down regulation of the Zeta chain in T cell is detectable in early breast cancer and correlates with immune cell function. Int. J. Cancer 131, 129–139 (2012)
G.P. Dunn, L.J. Old, R.D. Schreiber, The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21, 137–148 (2004)
R. Kim, M. Emi, K. Tanabe, K. Arihiro, Tumor driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 66, 527–536 (2006)
S. Ferrari, F. Malugani, B. Rovati, C. Porta, A. Riccardi, M. Danova, Flow cytometric analysis of circulating dendritic cells subsets and intracellular cytokine production in advanced breast cancer patients. Oncol. Rep. 14, 113–120 (2005)
M. Siddiqui, K. Ristow, S.N. Markovic, T.E. Witzig, T.M. Habermann, J.P. Colgan, D.J. Inwards, W.L. White, S.M. Ansell, I.N. Micallef, P.B. Johnston, T.G. Call, L.F. Porrata, Absolute lymphocyte count predicts overall survival in follicular lymphomas. Br. J. Haematol. 134, 596–601 (2006)
C. Borg, I. Ray-Coquard, I. Philip, G. Clapisson, N. Bendriss-Vermare, C. Menetrier-Caux, C. Sebban, P. Biron, J.Y. Blay, CD4 lymphopenia as a risk factor for febrile neutropenia and early death after cytotoxic chemotherapy in adult patients with cancer. Cancer 101, 2675–2680 (2004)
I. Ray-Coquard, H. Ghesquiere, T. Bachelot, C. Borg, P. Biron, C. Sebban, A. LeCesne, F. Chauvin, J.Y. Blay, ELYPSE Study Group, Identification of patients at risk for early death after conventional chemotherapy in solid tumors and lymphomas. Br. J. Cancer 85, 816–822 (2001)
Y. Oki, K. Yamamoto, H. Kato, Y. Kuwatsuka, H. Taji, Y. Kagami, Y. Morishima, Low absolute lymphocyte count is a poor prognostic marker in patients with diffuse large B-cell lymphoma and suggests patients’ survival benefit from rituximab. Eur. J. Haematol. 81, 1209–1215 (2008)
J. Péron, C. Cropet, O. Tredan, T. Bachelot, I. Ray-Coquard, G. Clapisson, S. Chabaud, I. Philip, C. Borg, P. Cassier, I. Labidi Galy, C. Sebban, D. Perol, P. Biron, C. Caux, C. Menetrier-Caux, J.Y. Blay, CD4 lymphopenia to identify and-of-life metastatic cancer patient. Eur. J. Cancer 49, 1080–1089 (2013)
I. Poschke, J. De Boniface, Y. Mao, R. Kiessling, Tumor-induced changes in the phenotype of blood-derived and tumor-associated T cells of early stage breast cancer patients. Int. J. Cancer 131, 1611–1620 (2012)
E. Collovà, B. Rovati, D. Grasso, K. Bencardino, M. Manzoni, M. Danova, Effect of peg-Filgrastim-supported dose-dense adjuvant chemotherapy on the peripheral blood leukocyte phenotype in breast cancer patients. Mol. Med. Rep. 2, 85–88 (2009)
C.L. Mackall, T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem Cells 18, 10–18 (2000)
H.F. Sewell, C.F. Halbert, R.A. Robins, A. Galvin, S. Chan, R.W. Blamey, Chemotherapy induced differential changes in lymphocyte subsets and natural killer cell function in patients with advanced breast cancer disease. Int. J. Cancer 55, 735–738 (1993)
E. Sara, A. Kotsakis, J. Souklekos, C. Kourousis, S. Kakolyris, E. Mavromanolakis, J. Vlachonicolis, V. Georgoulias, Post-chemotherapy lymphopoiesis in patients with solid tumors is characterized by CD4+ cell proliferation. Anticancer Res. 19, 471–476 (1999)
W. Schroeder, A. Vering, M. Stegmuller, R. Strohmeier, Lymphocyte subsets in patients with ovarian and breast cancer. Eur. J. Gynaecol. 18, 474–479 (1997)
B. Melichar, M. Touskova, J. Dvorak, P. Jandik, O. Kopecky, The peripheral blood leukocyte phenotype in patients with breast cancer: effect of doxorubicin/paclitaxel combination chemotherapy. Immunopharmacol. Immunotoxicol. 23, 163–173 (2001)
S. Ferrari, B. Rovati, L. Cucca, C. Scarabelli, M. Presti, C. Beccaria, E. Collovà, C. Porta, M. Danova, Impact of the topotecan-based chemotherapy on the immune system of advanced ovarian cancer patients: an immunophenotypic study. Oncol. Rep. 9, 1107–1113 (2002)
O.T. Chan, K.X. Yang, The immunological effects of taxanes. Cancer Immunol. Immunother. 49, 181–185 (2000)
A. Pinzon-Charry, C.S.K. Ho, R. Laherty, T. Maxwell, D. Walker, R.A. Gardiner, L. O’Connor, C. Pyke, C. Schmidt, C. Furnival, J.A. López, A population of HLA-DR+ immature cells accumulates in the blood dendritic cells compartment of patients with different types of cancer. Neoplasia 7, 1112–1122 (2005)
S.I. Labidi-Galy, V. Sisirak, P. Meeus, M. Gobert, I. Treilleux, A. Bajard, J.D. Combes, J. Faget, F. Mithieux, A. Cassignol, O. Tredan, I. Durand, C. Ménétrier-Caux, C. Caux, J.Y. Blay, I. Ray-Coquard, N. Bendriss-Vermare, Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res. 71, 5423–5434 (2011)
D.I. Gabrilovich, I.F. Ciernik, D. Carbone, Dendritic cells in antitumor immune responses. I defective antigen presentation in tumor-bearing hosts. Cell. Immunol. 170, 101–110 (1996)
S. Della Bella, M. Gennaro, M. Vaccari, C. Ferraris, S. Nicola, A. Riva, M. Clerici, M. Greco, M.L. Villa, Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br. J. Cancer 89, 1463–1472 (2003)
B. Almand, J.R. Resser, B. Lindman, S. Nadaf, J.I. Clark, E.D. Kwon, D.P. Carbone, D.I. Gabrilovich, Clinical significance of defective dendritic cell differentiation in cancer. Clin. Cancer Res. 6, 1755–1766 (2000)
G. Absenger, J. Szkandera, M. Stotz, M. Pichler, T. Winder, T. Langsenlehner, U. Langsenlehner, H. Samonigg, W. Renner, A. Gerger, A common and functional gene variant in the vascular endothelial growth factor a predicts clinical outcome in early-stage breast cancer. Mol. Carcinog. 52, E96–E102 (2013)
P. Mancuso, A. Burini, G. Pruneri, A. Goldhirsch, G. Martinelli, F. Bertolini, Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 97, 3658–3661 (2001)
L.V. Beerpoot, N. Mehra, J.S. Vermaat, B.A. Zonnenberg, M.F. Gebbink, E.E. Voest, Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann. Oncol. 15, 139–145 (2004)
G. Furstenberger, R. von Moos, R. Lucas, B. Thürlimann, H.J. Senn, J. Hamacher, E.M. Boneberg, Circulating endothelial cells and angiogenic serum factor during neoadjuvant chemotherapy of primary breast cancer. Br. J. Cancer 94, 524–531 (2006)
M.D. Groves, K.R. Hess, V.K. Puduvalli, H. Colman, C.A. Conrad, M.R. Gilbert, J. Weinberg, M. Cristofanilli, W.K. Yung, T.J. Liu, Biomarkers of disease: cerebrospinal fluid vascular endothelial growth factor (VEGF) and stromal cell derived factor (SDF)-1 levels in patients with neoplastic meningitis (NM) due to breast cancer, lung cancer and melanoma. J. Neurooncol. 94, 229–234 (2009)
G. Lazennec, A. Richmond, Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol. Med. 16, 133–144 (2000)
L.V. Rodhes, M.R. Bratton, Y. Zhu, S.L. Tilghman, S.E. Muir, V.A. Salvo, C.R. Tate, S. Elliott, K.P. Nephew, B.M. Collins-Burow, M.E. Burow, Effects of SDF-1-CXCR4 signalling on microRNA expression and tumorigenesis in estrogen receptor-alpha (ER-α)-positive breast cancer cells. Exp. Cell Res. 317, 2573–2581 (2011)
A. Muller, B. Homey, H. Soto, N. Ge, D. Catron, M.E. Buchanan, T. McClanahan, E. Murphy, W. Yuan, S.N. Wagner, J.L. Barrera, A. Mohar, E. Verástegui, A. Zlotnik, Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001)
M.C. Smith, K.E. Luker, J.R. Garbow, J.L. Prior, E. Jackson, D. Piwnica-Worms, G.D. Luker, CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 64, 8604–8612 (2004)
V. Ludovini, S. Gori, M. Colozza, L. Pistola, E. Rulli, I. Floriani, E. Pacifico, F.R. Tofanetti, A. Sidoni, C. Basurto, A. Rulli, L. Crinò, Evaluation of serum HER2 extracellular domain in early breast cancer patients: correlation with clinicopathological parameters and survival. Ann. Oncol. 19, 883–890 (2008)
V. Rossi, I. Sarotto, F. Maggiorotto, P. Berchialla, F. Kubatzki, N. Tomasi, S. Redana, R. Martinello, G. Valabrega, M. Aglietta, R. Ponzone, F. Montemurro, Moderate immunohistochemical expression of HER-2 (2+) without HER- 2 gene amplification is a negative prognostic factor in early breast cancer. Oncologist 17, 1418–1425 (2012)
R. Colomer, S. Montero, A. Lluch, B. Ojeda, A. Barnadas, A. Casado, B. Massutí, H. Cortés-Funes, B. Lloveras, Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin. Cancer Res. 6, 2356–2362 (2000)
D. Lüftner, P. Henschke, B. Flath, C. Akrivakis, S. Schnabel, B. Prinz, R. Geppert, K.D. Wernecke, K. Possinger, Serum HER2 /neu as a prediction and monitoring parameter in a phase II study with weekly paclitaxel in metastatic breast cancer. Anticancer Res. 24, 895–906 (2004)
L.N. Harris, V. Liotcheva, G. Broadwater, M.J. Ramirez, P. Maimonis, S. Anderson, T. Everett, D. Harpole, M.B. Moore, D.A. Berry, D. Rizzeri, J.J. Vredenburgh, R.C. Bentley, Comparison of methods of measuring HER-2 in metastatic breast cancer patients treated with high-dose chemotherapy. J. Clin. Oncol. 19, 1698–1706 (2001)
A. Lipton, S.M. Ali, K. Leitzel, L. Demers, V. Chinchilli, L. Engle, H.A. Harvey, C. Brady, C.M. Nalin, M. Dugan, W. Carney, J. Allard, Elevated serum Her-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J. Clin. Oncol. 20, 1467–1472 (2002)
G.B. Cook, I.E. Neaman, J.L. Goldblatt, D.R. Cambetas, M. Hussain, D. Lüftner, K.K. Yeung, D.W. Chan, M.K. Schwartz, W.J. Allard, Clinical utility of serum HER-2/neu testing on the bayer immuno 1 automated system in breast cancer. Anticancer Res. 21, 1465–1470 (2001)
F.J. Esteva, V. Valero, D. Booser, L.T. Guerra, J.L. Murray, L. Pusztai, M. Cristofanilli, B. Arun, B. Esmaeli, H.A. Fritsche, N. Sneige, T.L. Smith, G.N. Hortobagyi, Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J. Clin. Oncol. 20, 1800–1808 (2002)
R.R. Mehta, J.H. McDermott, T.J. Hieken, K.C. Marler, M.K. Patel, L.D. Wild, T.K. Das Gupta, Plasma c-erbB-2 levels in breast cancer patients: prognostic significance in predicting response to chemotherapy. J. Clin. Oncol. 16, 2409–2416 (1998)
I. Gruber, N. Landenbergen, A. Staebler, M. Hahn, D. Wallwiener, T. Fehm, Relationship between circulating tumor cells and peripheral T cells in patients with primary breast cancer. Anticancer Res. 33, 2233–2338 (2013)
A. Lucci, C.S. Hall, A.K. Lodhi, A. Bhattacharyya, A.E. Anderson, L. Xiao, I. Bedrosian, H.M. Kuerer, S. Krishnamurthy, Circulating tumor cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 13, 688–695 (2012)
V. Mikulova, M. Cabinakova, I. Janatkova, O. Mestek, T. Zima, P. Tesarova, Detection of circulating tumor cells during follow-up of patients with early breast cancer: clinical utility for monitoring of therapy efficacy. Scand. J. Clin. Lab. Invest. 19, 1–11 (2013)
T. Fleitas, V. Martinez-Sales, V. Vila, E. Reganon, D. Mesado, M. Martín, J. Gómez-Codina, J. Montalar, G. Reynés, Circulating endothelial cells and microparticle as prognostic markers in advanced non-small cell lung cancer. PLoS ONE 7, e47365 (2012)
P. Mancuso, F. Bertolini, Circulating endothelial cells as biomarkers in clinical oncology. Microvasc. Res. 79, 224–228 (2010)
B. Dome, J. Timar, J. Dobos, L. Meszaros, E. Raso, S. Paku, I. Kenessey, G. Ostoros, M. Magyar, A. Ladanyi, K. Bogos, J. Tovari, Identification and clinical significance of circulating endothelial progenitors cells in human non-small cell lung cancer. Cancer Res. 66, 7341–7347 (2006)
R.P. Naik, D. Jin, E. Chuang, E.G. Gold, E.A. Tousimis, A.L. Moore, P.J. Christos, T. de Dalmas, D. Donovan, S. Rafii, L.T. Vahdat, Circulating endothelial progenitors cells correlate to stage in patients with invasive breast cancer. Breast Cancer Res. Treat. 107, 133–138 (2008)
P.K. Goon, G.Y. Lip, P.S. Stonelake, A.D. Blann, Circulating endothelial cells and circulating progenitors cells in breast cancer: relationship to endothelial damage/dysfunction/apoptosis, clinicopathologic factors, and the Nottingham Prognostic Index. Neoplasia 11, 71–779 (2009)
D. Goodale, C. Phay, W. Brown, L. Gray-Statchuk, P. Furlong, M. Lock, I. Chin-Yee, M. Keeney, A.L. Allan, Flow cytometric assessment of monocyte activation markers and circulating endothelial cells in patients with localized or metastatic breast cancer. Cytometry B Clin. Cytom. 76, 107–117 (2009)
H.K. Kim, K.S. Song, H.O. Kim, J.H. Chung, K.R. Lee, D.H. Lee, E.S. Lee, H.K. Kim, K.W. Ryu, J.M. Bae, Circulating numbers of endothelial progenitors cells in patients with gastric and breast cancer. Cancer Lett. 198, 83–88 (2003)
C. Richter-Ehrenstaein, J. Rentzsch, S. Runkel, A. Schneider, G. Schonfelder, Endothelial progenitors cells in breast cancer patients. Breast Cancer Res. Treat. 106, 343–349 (2007)
Y.H. Kuo, C.H. Lin, W.Y. Shau, T.J. Chen, S.H. Yang, S.M. Huang, C. Hsu, Y.S. Lu, A.L. Cheng, Dynamics of endothelial cells and progenitors cells in breast cancer patients receiving cytotoxic chemotherapy. BMC Cancer 12, 620 (2012)
Y. Shaked, E. Henke, J.M. Roodhart, P. Mancuso, M.H. Langenberg, M. Colleoni, L.G. Daenen, S. Man, P. Xu, U. Emmenegger, T. Tang, Z. Zhu, L. Witte, R.M. Strieter, F. Bertolini, E.E. Voest, R. Benezra, R.S. Kerbel, Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 14, 263–267 (2008)
J.W. Ho, R.W. Pang, C. Lau, C.K. Sun, W.C. Yu, S.T. Fan, R.T. Poon, Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. J. Hepatol. 44, 836–843 (2006)
Acknowledgments
We wish to thank all the patients and volunteers for taking part in the study, as well as the nursing and the medical staff of the Oncologic Unit at the Fondazione IRCCS Policlinico San Matteo, for their support in the acquisition of clinical samples. The authors thank Giorgia Testa and Sabrina Nigrisoli (SC Pediatria, Laboratorio di Immuno Allergologia, Fondazione IRCCS Policlinico S. Matteo, Pavia) for technical support and Paul Baines (Cardiff, UK) for English language support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
This work was supported by the Fondazione IRCCS Policlinico San Matteo, Pavia (Hospital Research grant n° 08067611 to P. Pedrazzoli) and “quota 5×1000 dell’imposta su reddito delle persone fisiche” designed for health research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1s
Flow cytometric dot plot panels show a representative example on the gating utilized for the identification of circulating CD4 T cell memory. Panel a: CD4 lymphocyte subset identified by anti-CD4 and -CD3 expression markers; Panel b: CD4 memory subset identified from anti-CD45RO expression marker versus SSC. Panel c: the CD4 central and effector memory subsets identified from anti-CD45RO and -CD62L expression markers. The population identified by CD4+CD45RO+ overlaps to the sum of population CD4+CD45RO+CD62L+ (central memory) and CD4+CD45RO+CD62L- (effector memory) defined by CD45RO and CD62L expression markers. (PPT 144 kb)
Fig. 2s
Flow cytometric dot plot panels show an example a staining of CD25 against FoxP3 in breast cancer patients as compared to healthy females. Panels a and d show CD4 lymphocyte subset defined from anti-CD3 and -CD4 expression markers. Panels b and e show the CD4+ CD25+cells subset identified from anti-CD4 and -CD25 expression markers; Panels c and f show the T regulatory cell subset (Treg) identified by anti-FoxP3 and -CD25 expression markers. The count of Treg subset, in breast cancer patients (Panel c) as in the healthy females (Panel f), is lower to 1%. This data suggest that the CD4+CD25+ cells in these patients, are activated and not therapy resistant T regulatory cells. (PPT 248 kb)
Rights and permissions
About this article
Cite this article
Rovati, B., Mariucci, S., Delfanti, S. et al. Simultaneous detection of circulating immunological parameters and tumor biomarkers in early stage breast cancer patients during adjuvant chemotherapy. Cell Oncol. 39, 211–228 (2016). https://doi.org/10.1007/s13402-015-0264-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-015-0264-2