Abstract
Purpose
The histone deacetylase (HDAC) inhibitor suberoylanilide hydroxamic acid (SAHA) has been reported to exhibit anticancer activities in various cancer cell types, but as yet there are few reports on the anticancer effects of SAHA in oral squamous cell carcinoma (OSCC)-derived cells and xenograft models.
Methods
The anti-proliferative and apoptotic activities of SAHA were assessed in human HSC-3 and HSC-4 (OSCC)-derived cell lines and JB6 normal mouse skin-derived epidermal cells using histone acetylation, soft agar colony formation, trypan blue exclusion, 4′-6-diamidino-2-phenylindole (DAPI) staining, Live/Dead viability/cytotoxicity and Western blot analyses.
Results
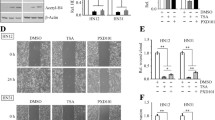
We found that SAHA treatment resulted in hyperacetylation of histones H2A and H3 and a concomitant decrease in the viability of HSC-3 and HSC-4 cells. SAHA also significantly inhibited the neoplastic transformation of JB6 cells treated with TPA, whereas the viability of these cells was not affected by this treatment. Additionally, we found that SAHA suppressed the anchorage-independent growth (colony forming capacity in soft agar) of HSC-3 and HSC-4 cells. DAPI staining, Live/Dead and Western blot analyses revealed that SAHA can induce caspase-dependent apoptosis in HSC-3 and HSC-4 cells. We also found that SAHA treatment led to inhibition of ERK phosphorylation, and that two MEK inhibitors potentiated SAHA-mediated apoptosis. Okadaic acid treatment inhibited SAHA-mediated apoptosis in both the HSC-3 and HSC-4 cell lines, wheras SAHA induced a profound in vivo inhibition of tumor growth in HSC-3 xenografts.
Conclusions
Our results indicate that the ERK signaling pathway may constitute a critical denominator of SAHA-induced apoptosis in OSCC-derived cells and that SAHA may have therapeutic potential for OSCC.







Similar content being viewed by others

References
C. Florean, M. Schnekenburger, C. Grandjenette, M. Dicato, M. Diederich, Epigenomics of leukemia: from mechanisms to therapeutic applications. Epigenomics 3, 581–609 (2011). doi:10.2217/epi.11.73
P. Zhu, E. Martin, J. Mengwasser, P. Schlag, K.P. Janssen, M. Gottlicher, Induction of HDAC2 expression upon loss of APC in colorectal tumorigenesis. Cancer Cell 5, 455–463 (2004)
Z. Zhang, H. Yamashita, T. Toyama, H. Sugiura, Y. Ando, K. Mita, M. Hamaguchi, Y. Hara, S. Kobayashi, H. Iwase, Quantitation of HDAC1 mRNA expression in invasive carcinoma of the breast. Breast Cancer Res. Treat. 94, 11–16 (2005). doi:10.1007/s10549-005-6001-1
E. Yiannakopoulou, Targeting epigenetic mechanisms and microRNAs by aspirin and other non steroidal anti-inflammatory agents–implications for cancer treatment and chemoprevention. Cell. Oncol. 37, 167–178 (2014). doi:10.1007/s13402-014-0175-7
B.H. Huang, M. Laban, C.H. Leung, L. Lee, C.K. Lee, M. Salto-Tellez, G.C. Raju, S.C. Hooi, Inhibition of histone deacetylase 2 increases apoptosis and p21Cip1/WAF1 expression, independent of histone deacetylase 1. Cell Death Differ. 12, 395–404 (2005). doi:10.1038/sj.cdd.4401567
K.B. Glaser, J. Li, M.J. Staver, R.Q. Wei, D.H. Albert, S.K. Davidsen, Role of class I and class II histone deacetylases in carcinoma cells using siRNA. Biochem. Biophys. Res. Comm. 310, 529–536 (2003)
P.A. Marks, The clinical development of histone deacetylase inhibitors as targeted anticancer drugs. Expert Opin. Investig. Drugs 19, 1049–1066 (2010). doi:10.1517/13543784.2010.510514
H. Yamamoto, J. Fujimoto, E. Okamoto, J. Furuyama, T. Tamaoki, T. Hashimoto-Tamaoki, Suppression of growth of hepatocellular carcinoma by sodium butyrate in vitro and in vivo. Int. J. Cancer 76, 897–902 (1998)
S.T. Nawrocki, J.S. Carew, L. Douglas, J.L. Cleveland, R. Humphreys, J.A. Houghton, Histone deacetylase inhibitors enhance lexatumumab-induced apoptosis via a p21Cip1-dependent decrease in survivin levels. Cancer Res. 67, 6987–6994 (2007). doi:10.1158/0008-5472.CAN-07-0812
D. Siegel, M. Hussein, C. Belani, F. Robert, E. Galanis, V.M. Richon, J. Garcia-Vargas, C. Sanz-Rodriguez, S. Rizvi, Vorinostat in solid and hematologic malignancies. J. Hematol. Oncol. 2, 31 (2009). doi:10.1186/1756-8722-2-31
D. Kurundkar, R.K. Srivastava, S.C. Chaudhary, M.E. Ballestas, L. Kopelovich, C.A. Elmets, M. Athar, Vorinostat, an HDAC inhibitor attenuates epidermoid squamous cell carcinoma growth by dampening mTOR signaling pathway in a human xenograft murine model. Toxicol. Appl. Pharmacol. 266, 233–244 (2013). doi:10.1016/j.taap.2012.11.002
G. Silva, B.A. Cardoso, H. Belo, A.M. Almeida, Vorinostat induces apoptosis and differentiation in myeloid malignancies: genetic and molecular mechanisms. PLoS One 8, e53766 (2013). doi:10.1371/journal.pone.0053766
M.N. Siddiquey, H. Nakagawa, S. Iwata, T. Kanazawa, M. Suzuki, K. Imadome, S. Fujiwara, F. Goshima, T. Murata, H. Kimura, Anti-tumor effects of suberoylanilide hydroxamic acid on Epstein-Barr virus-associated T cell and natural killer cell lymphoma. Cancer Sci. 105, 713–722 (2014). doi:10.1111/cas.12418
S. Chen, Y. Zhao, W.F. Gou, S. Zhao, Y. Takano, H.C. Zheng, The anti-tumor effects and molecular mechanisms of suberoylanilide hydroxamic acid (SAHA) on the aggressive phenotypes of ovarian carcinoma cells. PLoS One 8, e79781 (2013). doi:10.1371/journal.pone.0079781
C. Cortes, S.C. Kozma, A. Tauler, S. Ambrosio, MYCN concurrence with SAHA-induced cell death in human neuroblastoma cells. Cell. Oncol. 38, 341–352 (2015). doi:10.1007/s13402-015-0233-9
L. Ellis, R. Pili, Histone deacetylase inhibitors: advancing therapeutic strategies in hematological and solid malignancies. Pharmaceuticals 3, 2411–2469 (2010). doi:10.3390/ph3082441
D. Yin, J.M. Ong, J. Hu, J.C. Desmond, N. Kawamata, B.M. Konda, K.L. Black, H.P. Koeffler, Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res 13, 1045–1052 (2007). doi:10.1158/1078-0432.CCR-06-1261
P.N. Munster, T. Troso-Sandoval, N. Rosen, R. Rifkind, P.A. Marks, V.M. Richon, The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res. 61, 8492–8497 (2001)
J.A. Shin, G. Han, H.J. Kim, H.M. Kim, S.D. Cho, Chemopreventive and chemotherapeutic effect of a novel histone deacetylase inhibitor, by specificity protein 1 in MDA-MB-231 human breast cancer cells. Eur. J. Cancer Prev. 23, 277–285 (2014). doi:10.1097/CEJ.0000000000000041
A. Hrzenjak, F. Moinfar, M.L. Kremser, B. Strohmeier, E. Petru, K. Zatloukal, H. Denk, Histone deacetylase inhibitor vorinostat suppresses the growth of uterine sarcomas in vitro and in vivo. Mol. Cancer 9, 49 (2010). doi:10.1186/1476-4598-9-49
X. Sun, Z.S. Hasanali, A. Chen, D. Zhang, X. Liu, H.G. Wang, D.J. Feith, T.P. Loughran Jr., K. Xu, Suberoylanilide hydroxamic acid (SAHA) and cladribine synergistically induce apoptosis in NK-LGL leukaemia. Br. J. Haematol. 168, 371–383 (2015). doi:10.1111/bjh.13143
Y. Wang, W.Z. Kong, L.H. Xing, X. Yang, Effects and mechanism of suberoylanilide hydroxamic acid on the proliferation and apoptosis of human hepatoma cell line bel-7402. J. BUON 19, 698–704 (2014)
T.G. Lee, E.H. Jeong, S.Y. Kim, H.R. Kim and C.H. Kim, The combination of irreversible EGFR TKIs and SAHA induces apoptosis and autophagy-mediated cell death to overcome acquired resistance in EGFR T790M-mutated lung cancer. Int. J. Cancer (2014) doi:10.1002/ijc.29320
R.T. Allen, W.J. Hunter 3rd, D.K. Agrawal, Morphological and biochemical characterization and analysis of apoptosis. J. Pharmacol. Toxicol. Methods 37, 215–228 (1997)
Z. Jin, W.S. El-Deiry, Overview of cell death signaling pathways. Cancer Biol. Ther. 4, 139–163 (2005)
M. Suzuki, M. Endo, F. Shinohara, S. Echigo, H. Rikiishi, Enhancement of cisplatin cytotoxicity by SAHA involves endoplasmic reticulum stress-mediated apoptosis in oral squamous cell carcinoma cells. Cancer Chemother. Pharmacol. 64, 1115–1122 (2009). doi:10.1007/s00280-009-0969-x
Y.H. Kim, D.H. Lee, J.H. Jeong, Z.S. Guo, Y.J. Lee, Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochem. Pharmacol. 75, 1946–1958 (2008). doi:10.1016/j.bcp.2008.02.016
C.A. Hollmann, T. Owens, J. Nalbantoglu, T.J. Hudson, R. Sladek, Constitutive activation of extracellular signal-regulated kinase predisposes diffuse large B-cell lymphoma cell lines to CD40-mediated cell death. Cancer Res. 66, 3550–3557 (2006). doi:10.1158/0008-5472.CAN-05-2498
Y.J. Lee, H.N. Cho, J.W. Soh, G.J. Jhon, C.K. Cho, H.Y. Chung, S. Bae, S.J. Lee, Y.S. Lee, Oxidative stress-induced apoptosis is mediated by ERK1/2 phosphorylation. Exp. Cell Res. 291, 251–266 (2003)
F. Chang, L.S. Steelman, J.T. Lee, J.G. Shelton, P.M. Navolanic, W.L. Blalock, R.A. Franklin, J.A. McCubrey, Leukemia 17, 1263–1293 (2003). doi:10.1038/sj.leu.2402945
J.T. Lee Jr., J.A. McCubrey, Signal transduction mediated by the ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 16, 486–507 (2002). doi:10.1038/sj.leu.2402460
N.P. Judd, A.E. Winkler, O. Murillo-Sauca, J.J. Brotman, J.H. Law, J.S. Lewis Jr., G.P. Dunn, J.D. Bui, J.B. Sunwoo, R. Uppaluri, ERK1/2 regulation of CD44 modulates oral cancer aggressiveness. Cancer Res. 72, 365–374 (2012). doi:10.1158/0008-5472.CAN-11-1831
Y. Wang, S.Y. Wang, C.M. Hou, Y.J. Xu, Z.Y. Du and X.D. Yu, J. Histone deacetylase inhibitor SAHA induces inactivation of MAPK signaling and apoptosis in HL-60 cells. J. Exp. Hematol./Chin. Ass. Pathophysiol. 15, 267–271 (2007)
M.J. Nunes, M. Moutinho, I. Milagre, M.J. Gama and E. Rodrigues, J. Okadaic acid inhibits the trichostatin A-mediated increase of human CYP46A1 neuronal expression in a ERK1/2-Sp3-dependent pathway. Lipid Res. 53, 1910–1919 (2012) doi:10.1194/jlr.M027680
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A1A2055874), the Ministry of Science ICT & Future Planning (2014R1A4A1005309) and research funds of the Chonbuk National University in 2015.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Boonsil Jang and Ji-Ae Shin equally contributed as first author.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Jang, B., Shin, JA., Kim, YS. et al. Growth-suppressive effect of suberoylanilide hydroxamic acid (SAHA) on human oral cancer cells. Cell Oncol. 39, 79–87 (2016). https://doi.org/10.1007/s13402-015-0255-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-015-0255-3