Abstract
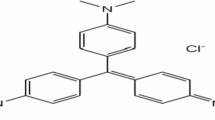
In this study, a renewable and effective bio-adsorbent was derived from Malaysian durian seeds (DSs) to act as a promising biosorbent for phytoremediation application towards removal of a hazardous cationic dye (crystal violet, CV) from aqueous environments. The physiochemical characteristics of DS were investigated by several analytical methods such as FTIR, TGA-DTG, BET, pHpzc, and SEM-EDX. Subsequently, a statistical optimization for CV removal by DS was carried out via Box-Behnken design (BBD) and numerical desirability function. In this regard, four operational factors that affect CV adsorption, i.e., DS dosage (0.02–0.1 g), initial pH (4–10), temperature (25–50 °C), and adsorption time (5–25 min) were optimized by BBD and numerical desirability function. Hence, the highest CV removal (93.91%) was recorded under the optimal conditions found through desirability function as follows: DS dosage of 0.081 g, solution pH = 9.9, working temperature = 34.6 °C, and contact time = 24.9 min. Furthermore, ANOVA test indicated the significant parametric interactions towards CV removal (%) can be observed between AB (DS dose vs. initial pH), AD (DS dose vs. time), and BC (initial pH vs. temperature) interactions. The adsorption kinetic process was well described by a pseudo-second-order model. Subsequently, the adsorption equilibrium isotherm was well presented by Freundlich and Temkin isotherm models with maximum adsorption capacity of 158 mg/g. Thus, the thermodynamic functions revealed that the adsorption process was spontaneous and endothermic in nature. The adsorption mechanism of CV on the DS surface can be ascribed to the electrostatic forces, n-π stacking, and H-bonding interactions. Thus, the output of the research work indicates the potential applicability of DS as a renewable and effective biosorbent for the removal of CV from aqueous environments.







Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request
References
Jawad AH, Kadhum AM, Ngoh YS (2018) Applicability of dragon fruit (Hylocereus polyrhizus) peels as low-cost biosorbent for adsorption of methylene blue from aqueous solution: kinetics, equilibrium and thermodynamics studies. Desalin. Water Treat. 109:231–240. https://doi.org/10.5004/dwt.2018.21976
Al-Tohamy R, Ali SS, Li F, Okasha KM, Mahmoud YAG, Elsamahy T, Sun J (2022) A critical review on the treatment of dye-containing wastewater: ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol Environ Safe 231:113160–113177. https://doi.org/10.1016/j.ecoenv.2021.113160
Javaid R, Qazi UY, Ikhlaq A, Zahid M, Alazmi A (2021) Subcritical and supercritical water oxidation for dye decomposition. J Environ Manage. 290:112605–112618. https://doi.org/10.1016/j.jenvman.2021.112605
Kahsay MH (2021) Synthesis and characterization of ZnO nanoparticles using aqueous extract of Becium grandiflorum for antimicrobial activity and adsorption of methylene blue. Appl Water Sci 11(2):1–12. https://doi.org/10.1007/s13201-021-01373-w
Allouss D, Essamlali Y, Amadine O, Chakir A, Zahouily M (2019) Response surface methodology for optimization of methylene blue adsorption onto carboxymethyl cellulose-based hydrogel beads: adsorption kinetics, isotherm, thermodynamics and reusability studies. RSC Adv 9(65):37858–37869. https://doi.org/10.1039/C9RA06450H
Mittal J (2020) Permissible synthetic food dyes in India. Resonance 25(4):567–577. https://doi.org/10.1007/s12045-020-0970-6
Rashad S, Zaki AH, Farghali AA (2019) Morphological effect of titanate nanostructures on the photocatalytic degradation of crystal violet. Nanomater Nanotechnol 9:1–10. https://doi.org/10.1177/1847980418821778
Mittal A, Mittal J, Malviya A, Kaur D, Gupta VK (2010) Adsorption of hazardous dye crystal violet from wastewater by waste materials. J colloid Inter Sci 343(2):463–473. https://doi.org/10.1016/j.jcis.2009.11.060
Mittal J, Ahmad R, Ejaz MO, Mariyam A, Mittal A (2022) A novel, eco-friendly bio-nanocomposite (Alg-Cst/Kal) for the adsorptive removal of crystal violet dye from its aqueous solutions. Int J Phyt 24(8):796–807. https://doi.org/10.1080/15226514.2021.1977778
He Q, Tan C, Zhang H (2017) Recent advances in cantilever-free scanning probe lithography: high-throughput, space-confined synthesis of nanostructures and beyond. ACS Nano 11(5):4381–4386. https://doi.org/10.1021/acsnano.7b03143
Patel A, Soni S, Mittal J, Mittal A, Arora C (2021) Sequestration of crystal violet from aqueous solution using ash of black turmeric rhizome. Desalin Water Treat 220:342–52. https://doi.org/10.5004/dwt.2021.26911
Soni S, Bajpai PK, Bharti D, Mittal J, Arora C (2020) Removal of crystal violet from aqueous solution using iron based metal organic framework. Desalin Water Treat 205:386–399. https://doi.org/10.5004/dwt.2020.26387
Cinperi NC, Ozturk E, Yigit NO, Kitis M (2019) Treatment of woolen textile wastewater using membrane bioreactor, nanofiltration and reverse osmosis for reuse in production processes. J Clean Prod 223:837–848. https://doi.org/10.1016/j.jclepro.2019.03.166
Li Y, An Y, Zhao R, Zhong Y, Long S, Yang J, Zheng H (2022) Synergetic removal of oppositely charged dyes by co-precipitation and amphoteric self-floating capturer: mechanism investigation by molecular simulation. Chemosphere 296:134033–134042. https://doi.org/10.1016/j.chemosphere.2022.134033
Rajala K, Grönfors O, Hesampour M, Mikola A (2020) Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res 183:116045–116054. https://doi.org/10.1016/j.watres.2020.116045
Sinha AK, Sasmal AK, Pal A, Pal D, Pal T (2021) Ammonium phosphomolybdate [(NH4)3PMo12O40] an inorganic ion exchanger for environmental application for purification of dye contaminant wastewater. J Photochem Photobiol A: Chem 418:113427–113436. https://doi.org/10.1016/j.jphotochem.2021.113427
Nozad E, Marjani AP, Mahmoudian M (2022) A novel and facile semi-IPN system in fabrication of solvent resistant nano-filtration membranes for effective separation of dye contamination in water and organic solvents. Sep Puri Technol 282:120121–120135. https://doi.org/10.1016/j.seppur.2021.120121
Machado GR, Carleer R, Arada PM, Gryglewicz G, Maggen J, Haeldermans T, Yperman J (2020) Adsorption of Cibacron Yellow F-4G dye onto activated carbons obtained from peanut hull and rice husk: kinetics and equilibrium studies. Biomass Conver Bio 12:323–339. https://doi.org/10.1007/s13399-020-00699-w
Guo X, Jia J, Gao P, Zhang T, Zha F, Tang X, Zuo Z (2022) Flower-like FeMoO4@1T-MoS2 micro-sphere for effectively cleaning binary dyes via photo-Fenton oxidation. J Colloid Inter Sci 622:284–297. https://doi.org/10.1016/j.jcis.2022.04.113
Tee GT, Gok XY, Yong WF (2022) Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: a review. Environ Res. 212:113248–113268. https://doi.org/10.1016/j.envres.2022.113248
Munagapati VS, Wen HY, Vijaya Y, Wen JC, Wen JH, Tian Z, Raul Garcia J (2021) Removal of anionic (Acid Yellow 17 and amaranth) dyes using aminated avocado (Persea americana) seed powder: adsorption/desorption, kinetics, isotherms, thermodynamics, and recycling studies. Int J Phytoremediation 23(9):911–923. https://doi.org/10.1080/15226514.2020.1866491
Jawad AH, Abdulhameed AS, Bahrudin NN, Hum NNMF, Surip SN, Syed-Hassan SSA, Sabar S (2021) Microporous activated carbon developed from KOH activated biomass waste: surface mechanistic study of methylene blue dye adsorption. Water Sci Technol 84(8):1858–1872. https://doi.org/10.2166/wst.2021.355
Gupta VK, Agarwal S, Ahmad R, Mirza A, Mittal J (2020) Sequestration of toxic congo red dye from aqueous solution using ecofriendly guar gum/activated carbon nanocomposite. Int J Boil Macromol. 158:1310–1318. https://doi.org/10.1016/j.ijbiomac.2020.05.025
Mittal J (2021) Recent progress in the synthesis of layered double hydroxides and their application for the adsorptive removal of dyes: a review. J Environ Manage 295:113017–113057. https://doi.org/10.1016/j.jenvman.2021.113017
Mittal A, Mittal J (2015) Hen feather: a remarkable adsorbent for dye removal. Green chemistry for dyes removal from wastewater. Green Chemistry for Dyes Removal from Wastewater: Research Trends and Applications 409-457.https://doi.org/10.1002/9781118721001
Mittal J, Mariyam A, Sakina F, Baker RT, Sharma AK, Mittal A (2021) Batch and bulk adsorptive removal of anionic dye using metal/halide-free ordered mesoporous carbon as adsorbent. J Clean Prod 321:129060–129074. https://doi.org/10.1016/j.jclepro.2021.129060
Mariyam A, Mittal J, Sakina F, Baker RT, Sharma AK, Mittal A (2021) Efficient batch and fixed-bed sequestration of a basic dye using a novel variant of ordered mesoporous carbon as adsorbent. Arab J Chem 14(6):103186–103201. https://doi.org/10.1016/j.arabjc.2021.103186
Jawad AH, Abdulhameed AS, Hanafiah MAKM, ALOthman ZA, Khan MR, Surip SN, (2021) Numerical desirability function for adsorption of methylene blue dye by sulfonated pomegranate peel biochar: modeling, kinetic, isotherm, thermodynamic, and mechanism study. Korean J Chem Eng 38(7):1499–1509. https://doi.org/10.1007/s11814-021-0801-9
Abdulhameed AS, Jawad AH, Ridwan M, Khadiran T, Wilson LD, Yaseen ZM (2022) Chitosan/carbon-doped TiO2 composite for adsorption of two anionic dyes in solution and gaseous SO2 capture: experimental modeling and optimization. J Polym Environ 1-18. https://doi.org/10.1007/s10924-022-02532-z
Kumar V, Saharan P, Sharma AK, Umar A, Kaushal I, Mittal A, Rashad B (2020) Silver doped manganese oxide-carbon nanotube nanocomposite for enhanced dye-sequestration: isotherm studies and RSM modelling approach. Ceram Int 46(8):10309–10319. https://doi.org/10.1016/j.ceramint.2020.01.025
Pitchay T, Jawad AH, Johari IS, Sabar S (2022) Kinetics studies of metallic ions adsorption by immobilised chitosan. Sci Lett 16(1):137–148. https://doi.org/10.24191/sl.v16i1.15932
Mubarak A, Shazwani N, Sabar S, Jawad AH (2020) The study of commercial titanium dioxide (TiO2) degussa P25 for the adsorption of acidic dye. Sci Lett. 14(1):68–83. https://doi.org/10.24191/sl.v14i1.10607
Rivera-Utrilla J, Sánchez-Polo M, Gómez-Serrano V, Álvarez PM, Alvim-Ferraz MCM, Dias JM (2011) Activated carbon modifications to enhance its water treatment applications. An overview. J Hazard Mater 187:1–23. https://doi.org/10.1016/j.jhazmat.2011.01.033
Reddy MS, Nirmala V, Ashwini C (2017) Bengal Gram Seed Husk as an adsorbent for the removal of dye from aqueous solutions–batch studies. Arab J Chem 10:S2554–S2566. https://doi.org/10.1016/j.arabjc.2013.09.029
Sidiqua MA, Priya VS (2021) Removal of yellow dye using composite binded adsorbent developed using natural clay and activated carbon from sapindus seed. Bio Agri Biotechnol 33:101965-1–1973. https://doi.org/10.1016/j.bcab.2021.101965
Bukhari A, Ijaz I, Zain H, Gilani E, Nazir A, Bukhari A, Iram S (2022) Removal of eosin dye from simulated media onto lemon peel-based low cost biosorbent. Arab J Chem. 15(7):103873–103885. https://doi.org/10.1016/j.arabjc.2022.103873
Fazal T, Faisal A, Mushtaq A, Hafeez A, Javed F, Alaud DA, Rehman F (2021) Macroalgae and coal-based biochar as a sustainable bioresource reuse for treatment of textile wastewater. Biomass Conver Bio 11(5):1491–1506. https://doi.org/10.1007/s13399-019-00555-6
Gayathiri M, Pulingam T, Lee KT, Sudesh K (2022) Activated carbon from biomass waste precursors: factors affecting production and adsorption mechanism. Chemosphere 294:133764–133776. https://doi.org/10.1016/j.chemosphere.2022.133764
Elgarahy AM, Elwakeel KZ, Mohammad SH, Elshoubaky GA (2021) A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean Eng Technol 4:100209–100214. https://doi.org/10.1016/j.clet.2021.100209
Shalaby SM, Madkour FF, El-Kassas HY, Mohamed A, Elgarahy AM (2021) Green synthesis of recyclable iron oxide nanoparticles using Spirulina platensis microalgae for adsorptive removal of cationic and anionic dyes. Environ Sci and Pollut Res 28(46):65549–65572. https://doi.org/10.1007/s11356-021-15544-4
Liu F, Sai KCKV, Zhang W (2021) Conversion of spiky sweetgum tree (Liquidambar styraciflua) seeds as into bio-adsorbent: static and dynamic adsorption assessment. J Hazard Mater Adva 1:100001–100011. https://doi.org/10.1016/j.hazadv.2021.100001
Bhatta LKG, Venkatesh K, Kiran N, Gundanna SK, Bhatta UM (2021) Synthesis and characterization of activated carbon from Delonix regia seeds for CO2 adsorption. Energy Climate Change 2:100064–100072. https://doi.org/10.1016/j.egycc.2021.100064
Soliman KN, Moustafa AF, Aboud AA, Halim KSA (2019) Effective utilization of Moringa seeds waste as a new green environmental adsorbent for removal of industrial toxic dyes. J Mater Res Technol 8(2):1798–1808. https://doi.org/10.1016/j.jmrt.2018.12.010
Wang Q, Wang Y, Yang Z, Han W, Yuan L, Zhang L, Huang X (2022) Efficient removal of Pb (II) and Cd (II) from aqueous solutions by mango seed biosorbent. Chem Eng J Adv 11:100295–100305. https://doi.org/10.1016/j.ceja.2022.100295
Ezekoye OM, Akpomie KG, Eze SI, Chukwujindu CN, Ani JU, Ujam OT (2020) Biosorptive interaction of alkaline modified Dialium guineense seed powders with ciprofloxacin in contaminated solution: central composite, kinetics, isotherm, thermodynamics, and desorption. Int J Phytoremediation 22(10):1028–1037. https://doi.org/10.1080/15226514.2020.1725869
Berry SK (1980) Cyclopropene fatty acids in some Malaysian edible seeds and nuts: I Durian (Durio zibethinus Murr). Lipids 15(6):452–455. https://doi.org/10.1007/BF02534071
Manshor MR, Anuar H, Aimi MN, Fitrie MA, Nazri WW, Sapuan SM, Wahit MU (2014) Mechanical, thermal and morphological properties of durian skin fibre reinforced PLA biocomposites. Mater Design 59:279–286. https://doi.org/10.1016/j.matdes.2014.02.062
Lee MC, Koay SC, Chan MY, Pang MM, Chou PM, Tsai KY (2018) Preparation and characterization of durian husk fiber filled polylactic acid biocomposites. MATEC Web Conf 152:02007–02014. https://doi.org/10.1051/matecconf/201815202007
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of Reactive Blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: optimization, reusability, kinetic and equilibrium studies. J Magnet Magnet Mater 404:179–189. https://doi.org/10.1016/j.jmmm.2015.12.040
Sing KS (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57(4):603–619. https://doi.org/10.1351/pac198557040603
Kanjana K, Harding P, Kwamman T, Kingkam W, Chutimasakul T (2021) Biomass-derived activated carbons with extremely narrow pore size distribution via eco-friendly synthesis for supercapacitor application. Biomass Bioenergy 153:106206–106218. https://doi.org/10.1016/j.biombioe.2021.106206
Elgarahy AM, Elwakeel KZ, Elshoubaky GA, Mohammad SH (2019) Microwave-accelerated sorption of cationic dyes onto green marine algal biomass. Environ Sci Pollution Res 26(22):22704–22722. https://doi.org/10.1007/s11356-019-05417-2
Ahmad MA, Ahmad N, Bello OS (2015) Modified durian seed as adsorbent for the removal of methyl red dye from aqueous solutions. Appl Water Sci 5(4):407–423. https://doi.org/10.1007/s13201-014-0208-4
González-García P, Centeno TA, Urones-Garrote E, Ávila-Brande D, Otero-Díaz LC (2013) Microstructure and surface properties of lignocellulosic-based activated carbons. Appl Surf Science 265:731–737. https://doi.org/10.1016/j.apsusc.2012.11.092
Ahmad MA, Ahmad N, Bello OS (2014) Adsorptive removal of malachite green dye using durian seed-based activated carbon. Water Air Soil Pollut 225(8):1–18. https://doi.org/10.1007/s11270-014-2057-z
Elwakeel KZ, Elgarahy AM, Elshoubaky GA, Mohammad SH (2020) Microwave assist sorption of crystal violet and Congo red dyes onto amphoteric sorbent based on upcycled Sepia shells. J Environ Health Sci Eng 18(1):35–50. https://doi.org/10.1007/s40201-019-00435-1
Kutluay S, Temel F (2021) Silica gel based new adsorbent having enhanced VOC dynamic adsorption/desorption performance. Colloids Surf A: Physicochem Eng Asp 609:125848–125862. https://doi.org/10.1016/j.colsurfa.2020.125848
Jawad AH, Abdulhameed AS, Wilson LD, Hanafiah MAKM, Nawawi WI, ALOthman ZA, Rizwan Khan M, (2021) Fabrication of schiff’s base chitosan-glutaraldehyde/activated charcoal composite for cationic dye removal: optimization using response surface methodology. J Polym Environ 29(9):2855–2868. https://doi.org/10.1007/s10924-021-02057-x
Shooto ND (2020) Removal of toxic hexavalent chromium (Cr (VI)) and divalent lead (Pb (II)) ions from aqueous solution by modified rhizomes of Acorus calamus. Surf Interface 20:100624–100633. https://doi.org/10.1016/j.surfin.2020.100624
Mourabet M, El Rhilassi A, El Boujaady H, Bennani-Ziatni M, El Hamri R, Taitai A (2012) Removal of fluoride from aqueous solution by adsorption on Apatitic tricalcium phosphate using Box-Behnken design and desirability function. Appl Surf Sci 258(10):4402–4410. https://doi.org/10.1016/j.apsusc.2011.12.125
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Vet. Akad. Handl. 24:1–39
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124. https://doi.org/10.1016/S0923-0467(98)00076-1
Qin P, Chen D, Li M, Li D, Gao Y, Zhu S, Lu M (2022) Melamine/MIL-101 (Fe)-derived magnetic carbon nanotube-decorated nitrogen-doped carbon materials as sorbent for rapid removal of organic dyes from environmental water sample. J Mol Liq 359:119231–119241. https://doi.org/10.1016/j.molliq.2022.119231
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403. https://doi.org/10.1021/ja02242a004
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta physiochim URSS 12:327–356
Siddiqui MN, Chanbasha B, Al-Arfaj AA, Kon’kova T, Ali I, (2021) Super-fast removal of cobalt metal ions in water using inexpensive mesoporous carbon obtained from industrial waste material. Environ Technol Innov 21:101257–101271. https://doi.org/10.1016/j.eti.2020.101257
Hoseinzadeh H, Hayati B, Ghaheh FS, Seifpanahi-Shabani K, Mahmoodi NM (2021) Development of room temperature synthesized and functionalized metal-organic framework/graphene oxide composite and pollutant adsorption ability. Mater Res Bullet 142:111408–111419. https://doi.org/10.1016/j.materresbull.2021.111408
AL-Shehri, HS, Almudaifer E, Alorabi AQ, Alanazi, HS, Alkorbi AS, Alharthi FA (2021) Effective adsorption of crystal violet from aqueous solutions with effective adsorbent: equilibrium, mechanism studies and modeling analysis. Environmental Pollut Bio 33(1):214-226.https://doi.org/10.1080/26395940.2021.1960199
Dil EA, Ghaedi M, Ghaedi A, Asfaram A, Jamshidi M, Purkait MK (2016) Application of artificial neural network and response surface methodology for the removal of crystal violet by zinc oxide nanorods loaded on activate carbon: kinetics and equilibrium study. J Taiwan Inst Chem Eng 59:210–220. https://doi.org/10.1016/j.jtice.2015.07.023
Yusuff AS, Ajayi OA, Popoola LT (2021) Application of Taguchi design approach to parametric optimization of adsorption of crystal violet dye by activated carbon from poultry litter. Sci African 13:e00850–e00863. https://doi.org/10.1016/j.sciaf.2021.e00850
Mohanty K, Naidu JT, Meikap BC, Biswas MN (2006) Removal of crystal violet from wastewater by activated carbons prepared from rice husk. Ind Eng Chem Res 45(14):5165–5171. https://doi.org/10.1021/ie060257r
Jawad AH, Abdulhameed AS, Surip SN, Sabar S (2020) Adsorptive performance of carbon modified chitosan biopolymer for cationic dye removal: kinetic, isotherm, thermodynamic, and mechanism study. Int J Environ Anal Chem 28:1–15. https://doi.org/10.1080/03067319.2020.1807966
Sinha R, Kumar R, Abhishek K, Shang J, Bhattacharya S, Sengupta S, Sharma P (2022) Single-step synthesis of activated magnetic biochar derived from rice husk for hexavalent chromium adsorption: equilibrium mechanism, kinetics, and thermodynamics analysis. Groundwater Sustain Develop 18:100796–100809. https://doi.org/10.1016/j.gsd.2022.100796
Kumari B, Tiwary RK, Yadav M, Singh KMP (2021) Nonlinear regression analysis and response surface modeling of Cr (VI) removal from synthetic wastewater by an agro-waste Cocos Nucifera: Box-Behnken Design (BBD). Int J Phytoremediation 23(8):791–808. https://doi.org/10.1080/15226514.2020.1858399
Acknowledgements
The authors would like to thank the Faculty of Applied Sciences, Universiti Teknologi MARA, Shah Alam, for all the research facilities. The author (Zeid A. ALOthman) is grateful to the Researchers Supporting Project No. (RSP-2021/1), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Nur Aimi Jani: formal analysis, validation, data curation. Larbi Haddad: formal analysis, validation, data curation, writing — original. Ahmed Saud Abdulhameed: formal analysis, validation, data curation, writing — original. Ali H. Jawad: conceptualization, methodology, software, supervision, project administration, writing — review and editing. Zeid A. ALOthman: validation, funding acquisition. Zaher Mundher Yaseen; formal analysis.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jani, N.A., Haddad, L., Abdulhameed, A.S. et al. Modeling and optimization of the adsorptive removal of crystal violet dye by durian (Durio zibethinus) seeds powder: insight into kinetic, isotherm, thermodynamic, and adsorption mechanism. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03319-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03319-x