Abstract
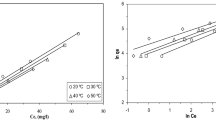
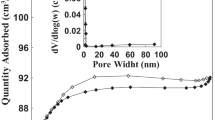
In this study, grape pulp (MGP) modified with NaOH and citric acid was used in the production of natural ion exchangers. The effects of parameters such as initial pH, MGP dosage, temperature, initial metal ion concentration, and contact time on the removal of Pb (II) and Cd (II) ions from aqueous solutions using modified materials were investigated by batch experiments. It was found that the experimental kinetic data fit the second-order model, and the activation energy for Pb (II) and Cd (II) adsorption processes were 20.68 and 38.61 kj mol−1, respectively. Although the initial adsorption rate increases with increasing temperature, the adsorption efficiency slightly decreases. It was calculated that the equilibrium data fit the Langmuir isotherm better, and the maximum adsorption capacities for Pb (II) and Cd (II) adsorption processes were approximately 1.496 and 1.022 mmol g−1 at 25 °C, respectively. Thermodynamic analysis has shown that the adsorption processes of Pb (II) and Cd (II) are exothermic (ΔH°Pb = −35.68 kj mol−1, ΔH°Cd = −21.19 kj mol−1) and have a self-developing character.
Graphical abstract










Similar content being viewed by others
References
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418 2011
Sheoran AS, Sheoran V (2006) Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Miner Eng 19:105–116 2006
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33:2469–2479
Renge VC, Khedkar SV, Pande SV (2012) Removal of heavy metals from wastewater using low cost adsorbents: a review. Sci Revs Chem Commun 2:580–584
Arslanoğlu H, Tümen F (2021) Potassium struvite (slow release fertilizer) and activated carbon production: resource recovery from vinasse and grape marc organic waste using thermal processing. Process Saf Environ 147(March):1077–1087
Arslanoğlu H (2019) Direct and facile synthesis of highly porous low cost carbon from potassium-rich wine stone and their application for high-performance removal. J Hazard Mater 374:238–247
Arslanoğlu H (2021) Production of low-cost asorbent with small particle size from calcium carbonate rich residue carbonatation cake and their high performance phosphate adsorption applications. J Mater Res Technol 11(March-April):428–447
Adelaja OA, Amoo IA, Aderibigbe AD (2011) Biosorption of Lead (II) ions from aqueous solution using Moringa oleifera pods. Arch Appl Sci Res 3:50–60
Abas SNA, Ismail MHS, Kamal ML, Izhar S (2013) Adsorption process of heavy metals by low-cost adsorbent: a review. World Appl Sci J 28:1518–1530
Arslanoglu H (2017) Removal of Cu (II) from aqueous solutions by using marble waste. Pamukkale Univ J Eng Sci 23(7):877–886
Yaraş A, Arslanoğlu H (2019) Utilization of paper mill sludge for removal of cationic textile dyes from aqueous solutions. Sep Sci Technol 54(16):2555–2566
Yaraş A, Arslanoğlu H (2018) Valorization of paper mill sludge as adsorbent in adsorption process of copper (II) ion from synthetic solution: kinetic, isotherm and thermodynamic studies. Arab J Sci Eng 43(5):2393–2402
Farooq U, Kozinski JA, Khan MA, Athar M (2010) Biosorption of heavy metal ions using wheat based biosorbents—a review of the recent literature. Bioresour Technol 101:5043–5053
Febrianto J, Kosasih AN, Sunarso J, Ju YH, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162:616–645
Göksungur Y, Üren S, Güvenç U (2005) Biosorption of cadmium and lead ions by ethanol treated waste baker’s yeast biomass. Bioresour Technol 96:103–109
Aksu Z, Isoglu A (2005) Removal of copper (II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochem 40:3031–3044
Dronnet VM, Renard CMGC, Axelos MAV, Thibault J-F (1997) Binding of divalent metalcations by sugar-beet pulp. Carbohydr Polym 34:73–82
Gerente C, Couespel du Mesnil P, Andres Y, Thibault J-F, Le Cloirec P (2000) Removal of metal ions from aqueous solution on low cost natural polysaccharides: sorption mechanism approach. React Funct Polym 46:135–144
Kartel MT, Kupchik LA, Veisov BK (1999) Evaluation of pectin binding of heavy metal ions in aqueous solutions. Chemosphere 38:2591–2596
Sag Y, Kutsal T (1996) The selective biosorption of chromium(VI) and copper(II) ions from binary metal mixtures by R. Arrhizus. Process Biochem 31:561–572
Veglio F, Beolchini F, Gasbarro A (1996) Biosorption of toxic metals an equilibrium study using free cells of Arthrobacter sp. Process Biochem 32:99–105
Zouboulis AI, Rousou EG, Matis KA, Hancock IC (1999) Removal of toxic metals from aqueous mixtures. Part 1: biosorption. J Chem Technol Biotechnol 74:429–436
Dronnet VM, Axelos MAV, Renard CMGC, Thibault J-F (1998) Improvoment of the binding capacity of metal cations by sugar-beet pulp. Part 1. Impact of cross-linking treatments on composition, hydration and binding properties. Carbohydr Polym 35:29–37
Eren MŞ, Arslanoğlu H, Çiftçi H (2020) Production of microporous Cu-doped BTC (Cu-BTC) metal-organic framework composite materials, superior adsorbents for the removal of methylene blue (Basic Blue 9). J Environ Chem Eng 8(5):104247
Marshall WE, Chatters AZ, Wartelle LH, McAloon A (2001) Optimization and estimated production cost of a citricacid-modified soybean hull ion exchanger. Ind Crop Prod 14:191–199
Altundogan HS, Arslan NE, Tumen F (2007) Copper removal from aqueous solutions by sugar beet pulp treated by NaOH and citric acid. J Hazard Mater 149:432–439
Arslanoglu H (2019) Adsorption of micronutrient metal ion onto struvite to prepare slow release multielement fertilizer: copper (II) doped-struvite. Chemosphere 217:393–401
Arslanoglu H, Tumen F (2012) A study on cations and color removal from thin sugar juice by modified sugar beet pulp. J Food Sci Technol 49:319–327
Arslanoğlu H, Orhan R, Turan MD (2019) Application of response surface methodology for the optimization of copper removal from aqueous solution by activated carbon prepared using waste polyurethane. Anal Lett 53(9):1343–1365
Sartape AS, Mandhare AM, Salvi PP, Pawar DK, Kolekar SS (2013) Kinetic and equilibrium studies of the adsorption of Cd(II) from aqueoussolutions by wood apple shell activated carbon. Desalin Water Treat 51:4638–4650
Dixit S, Singh DP (2013) Phycoremediation of lead and cadmium by employing Nostoc muscorum as biosorbent and optimization of its biosorption potential. Int J Phytoremediat 15:801–813
Khani MH (2013) Dynamics and thermodynamics studies on the lead and cadmium removal from aqueous solutions by Padina sp. Algae: studies in single and binary metal systems. Sep Sci Technol 48:2688–2699
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. K Sven Vetenskapsakad Handl 24:1–39
McKay G, Ho YS (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div, ASCE 89:31–60
Inglezakis VJ, Zorpas AA (2012) Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems. Desalin Water Treat 39:149–157
Freundlich H (1907) Ueber die Adsorption in Loesungen. Z Phys Chem 57:385–470
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Dubinin MM, Radushkevich LV (1947) Equation of the characteristic curve of activated charcoal. Proc Acad Sci Phys Chem Sect, USSR 55:331–333
Mahramanlioglu M, Kizilcikli I, Bicer IO (2002) Adsorption of fluoride from aqueous solution by acid treated spent bleaching earth. J Fluor Chem 115:41–47
El-Kamash AM, Zaki AA, El Geleel MA (2005) Modeling batch kinetics and thermodynamics of zinc and cadmium ions removal from waste solutions using synthetic zeolite A. J Hazard Mater 127:211–220
Arslanoglu H, Altundogan HS, Tumen F (2009) Heavy metals binding properties of esterified lemon. J Hazard Mater 164:1406–1413
Nagy B, Manzatu C, Aneanu AM, Indolean C, Silaghi-Dumitrescu L, Majdik C (2014) Effect of alkaline and oxidative treatment on sawdust capacity to remove Cd(II) from aqueous solutions: FTIR and AFM study. J Wood Chem Technol 34:301–311
Azouaou N, Sadaoui Z, Djaafri A, Mokaddem H (2010) Adsorption of cadmium from aqueou solutiononto untreated coffee grounds: equilibrium, kinetics and thermodynamics. J Hazard Mater 184:126–134
Liang S, Guo X, Feng N, Tian Q (2009) Adsorption of Cu(II) and Cd(II) fromaqueous solution by mercapto-acetic acid modified orange peel. Colloids Surf A Physicochem Eng Asp B73:10–14
Kelly-Vargas K, Cerro-Lopez M, Reyna-Tellez S, Bandala ER, Sanchez-Salas JL (2012) Biosorption of heavy metals in polluted water, using different waste fruit cortex. Phys Chem Earth 37–39:26–29
Senthil Kumar P, Ramalingam S, Abhinaya RV, Kirupha SD, Murugesan A, Sivanesan S (2012) Adsorption of metal ions onto the chemically modified agricultural waste. Clean-Soil Air Water 40:188–197
Ofomaja AE, Naidoo EB, Modise SJ (2010) Biosorption of Cu(II) and Pb(II) onto potassium hydroxide treated pine cone powder. J Environ Manag 91:1674–1685
Liu C, Ngo HH, Guo WS (2012) Water melon rind: agro-waste or superior biosorbent. Appl Biochem Biotechnol 167:1699–1715
Saka C, Sahin O, Demir H, Kahyaoglu M (2011) Removal of lead from aqueoussolutions using preboiled and formaldehyde treated onion skins as a newadsorbent. Sep Sci Technol 46:507–517
Ho YS, Wang CC (2004) Pseudo-isotherms for the sorption of cadmium ion onto tree fern. Process Biochem 39:761–765
Arslanoğlu H, Kaya S, Tümen F (2019) Cr (VI) adsorption on low-cost activated carbon developed from grape marc-vinasse mixture. Part Sci Technol 38(6):768–781
Feng N, Guo X, Liang S, Zhu Y, Liu J (2011) Biosorption of heavy metals fromaqueous solutions by chemically modified orange peel. J Hazard Mater 185:49–54
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Ion exchange resins were tested under typical and challenging conditions.
• Performance of resins was better than conventional resins.
• It has been ensured that Pb and Cd are effectively removed from waste water.
• pH 4.8 high Pb and Cd adsorption was obtained with the modified ion exchanger.
• Ion exchange resins can be reused to reduce process cost and byproduct production.
Rights and permissions
About this article
Cite this article
Arslanoğlu, E., Eren, M.Ş.A., Arslanoğlu, H. et al. Modification of grape pulp with citric acid for the production of natural ion exchanger resin and removal of Pb (II) and Cd (II) from aqueous solutions: kinetic, thermodynamics, and mechanism. Biomass Conv. Bioref. 13, 2349–2362 (2023). https://doi.org/10.1007/s13399-021-01521-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01521-x