Abstract
In this study, a series of seven residual biomass feedstock was treated by hydrothermal carbonization (HTC) at temperatures of 180 °C, 210 °C, 240 °C, and 270 °C and residence times of 0.5 h, 2 h, and 4 h. The processed samples were analyzed with focus on properties that are relevant for the combustion of a fuel. Temperature was found to have the highest impact on fuel properties. HTC has a positive effect on the energy density of the material, increasing lower heating values typically by 10–15% at 180 °C and 47–59% at 270 °C. At the same time, mass yield was decreasing for increasing treatment temperature. The hydrothermal treatment was found to have a profound impact on the inorganic composition of the fuels, lowering significantly the alkali metal and chlorine content while increasing silicon and phosphorous concentrations in the ash. These transformations lead to improvements in ash melting temperatures and in molar S/Cl ratio, an indicator commonly used to assess the risk of high-temperature corrosion in biomass combustion. HTC is also expected to have a positive impact on fine particle emissions upon combustion due to lowered concentrations of elements responsible for aerosol formation after HTC treatment. On the other hand, HTC leads to higher nitrogen contents in the fuel, thereby potentially increasing the risk for higher NOx emissions upon combustion of HTC-treated fuels. Overall, HTC clearly shows a positive effect on combustion properties, but the effects are fuel specific and especially interesting for biogenic waste that originates from lignocellulosic material. Applying the criteria of this study, the fuel properties of sewage sludge could not significantly be improved. For feedstock like this, the advantage of utilizing HTC as treatment lies in improved dewatering, storage, and feedstock logistics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Moving towards a more sustainable society has become one of the most important challenges for the twenty-first century. A major part of the worldwide greenhouse gas emissions originates from the energy production from fossil fuels. Biomass as an abundant, renewable energy carrier offers the possibility to reduce net greenhouse gas emissions by substituting fossil fuels [1, 2]. The energetic utilization of biomass, however, poses challenges caused by the huge diversity of these feedstocks. Unfavorable fuel properties, such as high alkali and chlorine content, lead to a variety of undesirable reactions in the furnaces of power boilers [3, 4]. Common problems in biomass combustion are ash related, such as corrosion, ash melting, deposit formation, and particle formation, as well as problems regarding nitrogen oxides (NOx), sulfur oxides (SOx), and hydrochloric acid (HCl) emissions. With an increasing demand for biofuels, also low-quality feedstocks and residues enter the market, further aggravating these issues [5, 6].
For example, firing of biomass in power boilers can lead to high-temperature corrosion of superheaters due formation of deposits by alkali chloride condensation and subsequent reaction of the superheater metal with chlorine (Cl) [4, 7]. To prevent high-temperature corrosion, alkali chloride formation needs to be suppressed. A possible process for this is the binding of alkali with sulfur (S) in the form of alkali sulfate in the combustion chamber [8]; a second option is to remove chlorine from the feedstock. Although the exact underlying reaction mechanisms of high-temperature corrosion are not yet fully understood, the prediction of the corrosion rate with the molar 2 S/Cl ration has proofed to be a useful indicator [9]. Only minor corrosion risks have to be expected for a molar 2 S/Cl ratio of > 4 [9].
Biomass combustion also leads to the emission of particulate matter (PM) that can cause respiratory problems and deposition on heat exchanger surfaces in the power plant. The two main sources of PM in biomass combustion are particles from incomplete combustion, such as soot, condensable organic matter, and char, and particles from ash-forming matter in the fuel [10, 11]. In the latter case of aerosol formation, a part of the volatile inorganics such as alkali metals, heavy metals, S, and Cl are released from the fuel to the gas phase during combustion and start to nucleate or condense [12]. Regarding biomass fuels, K, being often the most abundant inorganic species, is the most relevant component for aerosol emissions.
Additionally, NOx emissions are a possible pollutant resulting from the combustion of biomass. Generally, NOx emissions are contributing to rain acidification and the formation of smog and affect the tropospheric zone by facilitating ozone creation. The formation of NOx is known to originate mainly from the oxidation of fuel nitrogen and, to a lesser extent, from other mechanisms involving conversion of nitrogen from air [13,14,15].
One promising approach to address these challenges is biomass upgrading by hydrothermal carbonization (HTC). In this process, biomass is converted in water at temperatures of 150–300 °C and elevated pressure to a product called hydrochar [16, 17]. A number of reactions occur during HTC that fundamentally alter the structure of biomass by dehydration, decarboxylation, and cleavage of ester and ether bonds by hydrolysis [17]. Overall, these reactions lead to a solid product that has an increased energy density and higher hydrophobicity and is more brittle than the starting material [18,19,20]. Depending on the process conditions, the lower heating value of the fuel can be increased by up to 60%, mostly on the account of a higher carbon content of the hydrochars. The coalification of the material by HTC is governed by dehydration and decarboxylation. Higher reaction severity, i.e., higher temperature and residence time, leads to a higher degree of coalification and lower volatile content but also decreases mass yield due to dissolution of organic material in the water phase. Finding an effective and cost-efficient treatment of the resulting process water currently poses a challenge on the HTC technology, since persistent organic compounds form during the process. These compounds are hardly degraded during traditional wastewater treatment. Current developments on this issue are discussed in the literature [21,22,23,24].
Characteristic temperature regimes of HTC for lignocellulosic biomass are 150 °C where the decarboxylation of carbonyl groups starts and 180 °C, 200–230 °C, and 220–260 °C where hemicellulose, cellulose, and lignin degradation occurs [17, 25, 26]. Above temperatures of 250 °C more and more, liquid byproducts are formed, and mass yield decreases dramatically, marking the transition to hydrothermal liquefaction (HTL). The higher hydrophobicity and brittleness of the hydrochars are a result of the removal of oxygenated surface groups during the treatment.
HTC not only alters the structure of biomass but also largely affects the ash composition by leaching of inorganics to the process water. Yet, the extent to which inorganics can be removed from the feedstock by HTC is determined by the nature of the inorganic matter: Ash-forming elements in biomass exist in different forms, e.g., as soluble ions, organically associated, as included minerals or as excluded minerals [27, 28]. Soluble ions can easily be removed by washing of biomass in water already at ambient conditions and hence also by HTC [29]. However, two factors further promote the extraction of inorganics during HTC compared to simple water leaching: During HTC process, water is in a subcritical state, in which it exhibits a lower viscosity and higher solubility of organic substances than water under normal conditions [16]. Therefore, also inorganics that are organically associated can potentially be removed from the biomass matrix by ion exchange followed by the dissolution of the soluble salt. In addition, the disintegration of the biomass structure by HTC further facilitates the removal of inorganic matter. Only few studies have investigated the effect of HTC on the fate of inorganics. Reza et al. [30] have investigated the influence of HTC temperature on the ash composition of corn stover, miscanthus, switch grass, and rice hulls. They found that calcium, magnesium, sulfur, phosphorous, and potassium can be reduced during HTC, which they linked to the decomposition of hemicellulose. Silicon is only partly removed at higher temperatures. Smith et al. [31] have investigated the influence of feedstock on the combustion behavior of hydrochar. They observed a significant reduction of alkali metals. Smith et al. [32] have also investigated the influence of residence time on the inorganic composition of miscanthus and concluded that the extraction of some inorganics like sulfur and phosphorous is decreasing with increasing residence time. They confirmed that also for miscanthus, more than 75% of alkali metals are removed from the feedstock after HTC treatment.
The transformation of both the organic and inorganic components of the feedstock during HTC leads to changes in the combustion behavior. To evaluate whether these transformations caused by a HTC lead to an improvement of the fuel quality, the implications that these changes have on challenges in biomass combustion have to be assessed. In most cases, fuel indices derived from fuel analysis have been used for this purpose. Most researchers rely on indices and correlations that have been developed for coal. Yet, due to the inherently different composition and structure of biomass compared to coal, their usefulness for the assessment of biofuels is questionable [5, 6]. Few attempts have been made to develop new indices for the prediction of undesired furnace reactions for biomass fuels: Sommersacher et al. [6] have performed lab- and real-scale combustion tests of a large variety of biomass fuels. They evaluated fuel indices derived from chemical fuel analysis regarding their applicability for biomass combustion by comparison of the index prediction to their measurements using statistical analysis. This way they identified that, for instance, the molar 2 S/Cl ratio is a suitable indicator for corrosion risk. The N content in the fuel is an indicator for NOx emissions, and the sum of the concentrations of K, Na, Zn, and Pb can predict aerosol emissions. Näzelius et al. [5] have shown that for the estimation of slagging in fixed-bed combustion of phosphorous-poor biomass fuels, none of the traditional fuel indices performs well. Instead, they proposed the use of a ternary diagram with K2O (+Na2O), CaO (+MgO), and SiO2 as crucial components to predict slagging behavior.
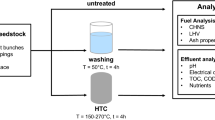
In this work for the first time, a large number of seven different waste feedstocks have been hydrothermally treated at three different residence times and four different temperatures. The aim of this study is to provide an overview of the impact of HTC on the fuel quality regarding combustion-related issues. The study focuses on the change in inorganic composition of HTC-treated fuels. However, it also considers risk of pollutant emission from, i.e., NOx and fine particles that to date are hardly discussed in literature. The feedstock includes agricultural residues such as straw and empty fruit bunches, forestry residues like bark, and residues from food production such as olive pomace. Additionally, digestate as a secondary product from energy production and grass cuttings and sewage sludge representing typical municipal wastes are investigated. Only fuel indices that have been proven suitable for the assessment of biomass-derived fuels are applied. The impact of HTC on energy density, ash composition, ash melting, corrosion tendency, NOx emissions, and fine particle formation is evaluated.
2 Materials and methods
2.1 Materials
Seven different biomass feedstocks are investigated in this study. Digestate was obtained from a local biogas plant digesting corn silage; empty fruit bunches were imported from Malaysia. Lawn cuttings were obtained from a local composting facility; olive pomace was obtained from south France. Sewage sludge was collected from a local wastewater facility, and wheat straw was provided from the Netherlands. Spruce bark was obtained from a pulp and paper mill in Finland. Prior to HTC, all collected biomass samples were dried and milled to a particle size below 1 mm.
2.2 Hydrothermal carbonization and fuel characterization
HTC experiments were carried out in a stirred Parr mini batch reactor that has been described in previous work [33]. The reactor has a volume of 600 mL and can be operated at a temperature range of up to 350 °C and a pressure range of up to 200 bar. The reactor is electrically heated with three 700-W powered heating jackets and was pressurized with argon. The pressure in the system was kept constant throughout the process with a backpressure regulator. In all experiments, 30 g of dry biomass were submerged in 300 mL deionized water and poured into the reactor. The reactor was then pressurized and heated to reaction temperature at a heating rate of 7 K min−1 and held at the specified residence time. In this study, biomass was carbonized at temperatures and pressure of 180 °C/20 bar, 210 °C/40 bar, 240 °C/40 bar, and 270 °C/80 bar. Residence time of was varied from 0.5 h, 2 h, to 4 h. After the reaction, the reactor was cooled, and the slurry was filtered and dried at ambient conditions to isolate the solid product. Fuel analysis data is provided on a dry basis in all tables and figures.
2.3 Sample analysis
Proximate analysis of the raw materials and hydrochar was carried out according to industrial standard methods DIN 51718 for the moisture content, DIN 14775/EN14775:2009 for determination of ash content, and DIN 51720/ISO 562:1998 for the volatile content using a moisture analyzer Denver IR60 and a muffle furnace. The amount of fixed carbon was calculated by closing the mass balance. Ultimate analysis was done according to industrial standard method DIN 51732/ISO/CD 12902:2006-11 in an elemental analyzer Vario Marco Cube from elementar. The chlorine content was determined in a similar device vario MACRO cube, also from elementar. The heating value was determined according to DIN 51900-1/ISO 1928:1995 using a bomb calorimeter C 200 from IKA. Ash compositions were determined by X-ray fluorescence (XRF) after complete combustion of the samples at 550 °C according to DIN 14775/EN14775:2009. For ash composition analysis, the instrument of Shimadzu EDX-800 HS was used. The examination requirement for fuel ashes by means of compressed tablets according to DIN 14775/EN14775:2009 for the determination of ash composition was used in this case. The pellet contains 100 mg of the test ash and 20 mg of a binder, in this case wax. Ash melting temperatures were determined following the standard method DIN 15370-1/ISO 540:1995-03.
3 Results and discussion
3.1 Mass and energy yield and heating values
Biomass samples are treated at temperatures ranging from 180 to 270 °C at constant residence time of 2 h. The treated biomass is referred to as hydrochar. Table 1 lists the mass yield, the lower heating value (LHV), and elemental composition obtained at different treatment temperatures. For all investigated biomass types, the mass yield is constantly decreasing for higher treatment temperatures. At the same time, the LHV on dry basis is increasing significantly for all treatment temperatures above 180 °C. An exception to this is sewage sludge where the LHV is slightly decreased by HTC. The temperature range investigated covers a wide range of conditions with different predominant reaction mechanisms: Decarboxylation of unstable carboxylic acids, releasing CO2, occurs starting from 150 °C [17]. Generally, during HTC, a variety of different reactions occur that lead to the removal of oxygen and hydrogen in the fuel matrix mainly by decarboxylation and dehydration [16]. Consequently, the hydrochar has a higher carbon content, also leading to a higher energy content of the fuel. During HTC of lignocellulosic biomass, the composition changes with more severe reaction conditions, i.e., the higher treatment temperature. Significant reactions occur above 180 °C where hydrolysis of hemicellulose (180 °C), cellulose (220 °C), and lignin (180–220 °C) takes place [25]. On the other hand, treating sewage sludge by HTC does not lead to a significant energy densification on dry basis. The reason for this is that upon HTC treatment of sewage sludge, the ash content is increased from 21.8 wt.% to 55.8 wt.% when comparing the starting material with sewage sludge treated at 270 °C. On an ash-free basis, also the LHV of sewage sludge is increased 1.4-fold. The mass loss with higher reaction severity is attributed to the dissolution of organic material in the process water. Increasing LHV and decreasing mass yield is also observed with increasing temperature for both other residence times 0.5 h and 4 h. LHV further increases with longer residence times, but temperature remains the most influential process parameter for this property. Within the investigated residence time ranges to up to 4 h, mass yield slightly decreases for increasing residence times for all feedstock. The full dataset for residence times 0.5 h and 4 h can be found in the supplementary material. The obtained energy yield, i.e., the ratio of LHV of the HTC-treated and untreated fuel multiplied by the mass yield, does not diminish as strongly as the mass yield with increasing treatment temperature. The lowest energy yields were obtained for sewage sludge with only 45–55% recovered energy content after HTC. The other feedstock exhibits fairly similar energy yields of around 80% at the lowest treatment temperature of 180 °C, which decreases to around 60% at the highest treatment temperature of 270 °C.
3.2 Inorganic biomass composition
Figure 1 shows the ash composition of the raw biomass feedstock investigated in this study. Depending on its origin, functionality within the organism or usage history each biomass has a unique inorganic composition. Major ash-forming elements are silicon (Si), potassium (K), phosphorous (P), calcium (Ca), aluminum (Al), magnesium (Mg), sodium (Na), and iron (Fe). Ashes from digestate, lawn cuttings, and sewage sludge are rich in P. Empty fruit bunches and olive pomace show high concentrations of K. In spruce bark, Ca constitutes the most abundant inorganic species, whereas ash from wheat straw contains mostly Si. Further, also the ash content given in Table 1 is strongly feedstock dependent. Ash contents below 5 wt.% ash are found for untreated olive pomace with 2.01 wt.% and spruce bark with 3.43 wt.%. Untreated sewage sludge has the highest ash content with 28.1 wt.%. Digestate, empty fruit bunches, lawn cuttings, and wheat straw all have similar ash contents of 8.9 wt.%, 8.3 wt.%, 9.5 wt.%, and 10.8 wt.%, respectively.
After HTC for most biomass feedstocks investigated in this study, the ash content increases with increasing treatment temperature. During HTC, inorganics can be extracted from the biomass matrix to the process water and hence are removed from the resulting solid fuel. However, at the same time, also organic material is dissolved in the process water leading to a decreasing overall mass yield. Thus, if the loss of organic material is higher than the reduction of inorganic elements, the ash content increases after the pre-treatment. An increase in ash content after HTC is observed for biomass types containing high shares of Si and P like digestate, lawn cuttings, sewage sludge, and wheat straw. In contrast, HTC slightly reduces the ash content of olive pomace, spruce bark, and empty fruit bunches that are rich in K. Consequently, there seems to be a correlation between the raw biomass ash composition and the effect of HTC on the ash content of the treated biomass.
Besides, HTC has the capability of altering the ash composition upon treatment. The characteristics of water under hydrothermal conditions, i.e., low viscosity and low dielectrical constant, as well as the overall degradation of biomass during the process potentially facilitate extraction of ash-forming matter from the feedstock. Table 2 shows an assessment of the influence of HTC on the concentration of main ash-forming elements Na, K, Ca, Mg, Si, and P. The change in ash concentration is determined by calculating the ratio R of the element weight percentage in the ash of treated and untreated feedstock. Elements that decrease in ash concentration are denoted with a minus sign (R < 0.9). Elements that do not change significantly in concentration (0.9 < R < 1.1) are denoted with o. Elements that increase in ash concentration (R > 1.1) are marked with a plus sign. Table 2 shows that for all investigated feedstocks, K is removed during HTC, while Ca, Si, and P removal is limited, and, therefore, the resulting hydrochar ash is richer in Ca, Si, and P. Mg removal depends on feedstock: For most biomass feedstock, the ash of the resulting hydrochar is lower in Mg, while in case of digestate and olive pomace, Mg levels are increasing after HTC.
In addition, the efficiency of HTC in removing main ash elements Na, K, Ca, Mg, Si, and P from the feedstock was evaluated. The extent to which ash-forming elements can be removed from the feedstock by HTC is dependent on its chemical nature. Therefore, removal of inorganic constituents is dependent on feedstock and treatment conditions. Figure 2 illustrates the HTC removal efficiency regarding Na, K, Ca, Mg, Si, and P in the ash of HTC-treated digestate as a function of temperature at different residence time. The removal efficiency was calculated by subtracting the ratio of the inorganic element content of the hydrochar with the raw feedstock multiplied by the mass yield from 100%, as also applied by Smith et al. [31]. Figure 2 illustrates digestate as an example, yet the following observations are made for all feedstock: It appears that the treatment temperature has a stronger effect on the element concentrations than residence time. Alkali metals Na and K both are extracted to a large extend during HTC. Between 29 and 100% of Na are removed upon treatment at 180 °C. This fraction increases to above 35% for all feedstock at the highest treatment temperature of 270 °C. Already at 180 °C and 2 h residence time, between 57 and 90% of the initially present K is removed from the feedstock. At 270 °C, between 63 and 95% of K is extracted. Consequently, a further increase in temperature further enhances K removal by up to 30%. On the other hand, the impact of residence time is less pronounced: The differences in K removal at 210 °C, for instance, only vary between 2 and 6% when increasing the residence times from 0.5 to 4 h. Na and K in biomass are mostly present as a highly soluble ion such as sodium or potassium nitrate and chloride and, therefore, easily removed by HTC [27, 34]. With increasing temperatures, biomass degradation advances facilitating further the removal of Na and K. Ca is only removed to a limited extent during HTC. In plant-derived biomass, Ca mainly fulfills structural functions in the cell wall and has limited mobility [35]. It is present as calcium pectate or calcium oxalate crystals which both exhibit poor water solubility, explaining the increase of concentration of Ca species in the ash of HTC-treated biomass. For most investigated feedstock, temperature shows a stronger positive effect on removal efficiencies than residence time. For instance, spruce bark treated for 4 h Ca removal increases from 24% at 180 °C to 65% at 270 °C, while increasing the residence time from 0.5 h to 4 h only enhances Ca removal by 2 and 18% at 180 °C and 270 °C, respectively. For other biomass types, i.e., lawn cuttings or sewage sludge, no significant change in concentration was observed with increasing temperature and/or residence time. No clear trend is observed for Mg that is depending on the raw material, either enriched or removed: The Mg concentration in the ash is slightly increasing in the case of HTC-treated digestate and lawn cuttings; for all other feedstock, the concentration in the hydrochar ash is decreasing.
The Si concentration in the ash increases after HTC in all hydrochar samples. Typically only between 20 and 40% of the initially present Si are removed. A further increase in temperature and residence time does not lead to higher removal percentages. Si in plants is mostly present as stable hydrated oxides and is incorporated within the plant cell wall and, therefore, not accessible for removal [30, 36]. P is also accumulating in the hydrochar. Initially at temperatures below 210 °C, P is extracted from the feedstock. For instance, when treating olive pomace at 180 °C for 2 h, almost 87% of P is removed, at higher temperatures of 270 °C and 2 h; however, this fraction decreases to only 31%. Similar trends can be observed for empty fruit bunches, spruce bark, and wheat straw. For all other investigated feedstock, the P removal is limited to 0–30% and shows no strong correlation with neither treatment temperature nor residence time.
3.3 Ash melting behavior
In traditional ash melting analysis, the temperatures at which the various stages of ash softening and melting take place are assessed and described by four characteristic temperatures: initial deformation temperature (IDT), softening temperature (ST), hemispherical temperature (HT), and flow temperature (FT). It is a widely accepted method to predict the likelihood of deposition of ash particles on heat exchange surfaces. Despite high complexity of ash melting behavior, a number of inorganic biomass constituents have been shown to play an important role: While Mg and Ca generally increase ash melting temperatures, alkali metals are known to form low-temperature melting alkali silicates in the presence of Si. High P contents and high concentrations of chlorine are also often linked to low ash melting temperatures. Table 3 shows the ash melting temperatures of raw and HTC-treated biomass ash samples. Olive pomace samples were not analyzed due to the very low ash content. Biomass ashes from feedstock rich in P and K such as sewage sludge, digestate, and empty fruit bunches exhibit the lowest initial deformations at the temperatures of 772, 863, and 837 °C. Spruce bark, being rich in Ca, shows a higher IDT temperature of 1304 °C.
Concerning the impact of HTC on ash melting temperatures, the following observations can be made: The effect of HTC on the IDT seems to be quite variable. On the one hand for some biomass types, i.e., wheat straw, the effect of HTC seems to be highly beneficial increasing the IDT of the sample treated at 210 °C by over 130 °C. On the other hand for spruce bark and sewage sludge, the IDT surprisingly is lowered after the pre-treatment. The other characteristic of ash melting temperatures ST, HT, and FT shows a more consistent trend: they are increased for all feedstocks after a HTC treatment. A higher treatment temperature, however, does not necessarily further lead to significant improvement concerning ash melting temperatures. HTC fundamentally changes the composition of treated biomass samples. Improvements in ash melting after a pre-treatment can be explained by the removal elements lowering the ash melting temperature such as alkali metals or chlorine. On the other hand, elements beneficial for ash melting temperatures such as Ca and Mg are removed to a lesser extent and thereby accumulate in the hydrochar.
3.4 Corrosion tendency (molar 2 S/cl ratio)
Firing of biomass can lead to high-temperature corrosion of superheaters due to formation of deposits by alkali chloride condensation and subsequent reaction of the superheater metal with chlorine (Cl) [7, 37]. The prediction of the corrosion rate with the molar 2 S/Cl ration has proven to be a useful indicator [9]. Only minor corrosion risks have to be expected for a molar 2 S/Cl ratio of > 4 [9].
Figure 3 shows the evolution of the molar 2 S/Cl ratio of HTC-treated biomass with temperature of samples treated for 2 h. Overall the values of molar 2 S/Cl ratio are increasing with increasing HTC treatment temperature for digestate, empty fruit bunches, lawn cuttings, and wheat straw. For these feedstock already at a temperature of 180 °C, the molar 2 S/Cl ratio is increased by 130–780%. At a residence time of 2 h, further increase of HTC treatment temperature does not lead to a further increase of molar 2 S/Cl ratio. Data for 0.5 h and 4 h of residence time, however, show a further increase of molar 2 S/Cl ratio for higher treatment temperatures (see supplementary material). Overall, for 20% of all HTC-treated samples, the Cl content lies below the detection limit of 0.01 wt.% for our device. In these samples, Cl concentrations are so low that Cl-induced corrosion is highly unlikely during biomass combustion. On the other hand, for spruce bark and sewage sludge, a reduction of 2 S/Cl ratio is observed.
These changes in molar 2 S/Cl ratio are a consequence of different concentrations of Cl and S in the fuels. The majority of Cl in biomass is present in the form of water-soluble salt [38, 39]. At a temperature of 180 °C, the directly accessible Cl is dissolved leading to the improvements compared to the raw material. For temperatures > 180 °C, biomass degradation possibly exposes additional inorganic domains for salt dissolution and thereby additional Cl reduction. Thus, the molar 2 S/Cl ratio increases further for higher temperatures. As seen in Table 1, the S weight fraction in the solid fuel is not strongly affected by a HTC treatment. It seems a balance between S extraction, and mass loss of other organic material is maintained; thus, the weight fraction of S in the solid fuel stays approximately constant. In general, the values of molar 2 S/Cl ratio can be improved by HTC. This amelioration is attributed to the reduction of Cl in the fuel, based on leaching of Cl species to the process water.
3.5 Risk for nitrogen oxides emissions
Nitric oxide (NOx) emissions are a possible pollutant resulting from the combustion of biomass. The formation of NOx is known to originate mainly from the oxidation of fuel nitrogen and, to a lesser extent, from other mechanisms involving conversion of nitrogen from air [13,14,15]. Therefore, it is important to investigate the effect of an HTC pre-treatment on the fuel N content. Figure 4 shows the relative fuel N content of the produced hydrochar. The relative fuel N is defined as the ratio of the nitrogen content of the hydrochar and the raw material. A value above one indicates that after HTC, the nitrogen content increases. Figure 4 shows that for most feedstock, the relative fuel N content in the hydrochars is increased by between 10 and 40% compared to the starting material. Only for sewage sludge, the relative fuel N content is reduced by roughly 10–20%. The evolution of nitrogen content with temperature is feedstock dependent. While for empty fruit bunches and digestate, a higher treatment temperature leads to further increase in fuel N content, and for other feedstocks, quite ambiguous trends are observed.
Consequently, according to the relationship between fuel N content and NOx emission established by Sommersacher et al. [6], HTC treatment is expected to have an adverse effect on NOx emissions during combustion of the char due to increased fuel N content.
During HTC, nitrogen is released to the process water. The initial decline in fuel N at low temperatures can be explained by the removal of nitrogen in the form of water-soluble ammonium and nitrite salts. Also, hydrolysis leads to dissolution of nitrogen [40] in the process water, causing even higher removal of nitrogen for higher reaction temperatures. The apparent rise of N content in the solid residue is a consequence of higher losses of other organic material.
Another approach to assess the NOx emission risk is to consider the specific nitrogen content per unit energy content. Figure 5 depicts the development of specific nitrogen contents per unit energy in mg MJ−1 for all feedstock with increasing temperature for samples treated for 2 h. Compared to the starting material, the specific nitrogen content per unit energy is decreased by roughly 10–40% at a treatment temperature of 180 °C. Upon further increasing the treatment temperature, the nitrogen contents per unit energy slightly increase with increasing temperature, diminishing the initial reduction to only 20% on average. In the case of HTC-treated digestate for treatment temperatures above 210 °C, the value of specific nitrogen content per unit energy is exceeding the value obtained for the starting material. Again sewage sludge marks the exception for which the nitrogen contents per unit energy decreases further with increasing treatment temperature. The same observations on temperature dependence of specific nitrogen content per unit energy can be made analyzing the samples that have been treated at 0.5 h and 4 h. Hence, according to this indicator, HTC could have a positive effect on NOx emission risk. On the other hand, also the volatile matter of the fuels is reduced by HTC. This likely shifts the N partitioning between volatiles and char towards a higher fraction of N in the char, which in general reduces the ability of the volatiles to reduce NOx formation in situ [41].
The fuel analysis points towards a higher NOx emission risk for HTC-treated fuels; however, further experimental validation in combustion tests is required for a final conclusion.
3.6 Fine particle emissions
Biomass combustion leads to the emission of particulate matter (PM) that can cause respiratory problems and deposition on heat exchanger surfaces in the power plant. Regarding biomass fuels, K, being often the most abundant inorganic species, is the most relevant component for aerosol emissions. Sommersacher et al. [6] have shown that the sum of K, Na, Zn, and Pb in mg kg−1 fuel can serve as an indicator regarding aerosol emissions and deposit built-up on heat exchanger surfaces. Based on this index, low PM emissions are expected for values < 1000 mg kg−1, medium PM emissions for values between 1000 and 10.000 mg kg−1, and high emissions for values > 10.000 mg kg−1. The applicability of the index for P- and Si-rich fuels is limited due to their possible influence on the K-release. Table 4 shows the evolution of the concentration of aerosol-forming inorganics for the raw feedstock, as well as the feedstock treated at different temperatures and residence times.
For all investigated fuels, a drastic decline in the sum of K, Na, Zn, and Pb is observed after HTC treatment compared to the raw fuel. The improvement is a consequence of removal of K by dissolution of potassium salts in the process water. As discussed previously, K in biomass is mostly present in extractives and hemicellulose as highly water-soluble salt [4]. Therefore, it can effectively be removed by a HTC treatment, where additional K is solubilized from the biomass matrix at temperatures above 150 °C, where degradation of hemicellulose starts. The concentrations of Na and Zn only contribute to the sum of the element concentrations to a minor part: their concentrations typically lie below 1.000 mg kg−1 fuel. The strongest reduction in concentration is already achieved at low HTC temperatures since for higher temperatures, the higher ash content of the hydrochar is increasing which diminishes the positive effect of K removal. At temperatures above 240 °C, a concentration of aerosol-forming elements below 10.000 mg kg−1 is achieved for all fuels abating the amount of PM emissions.
4 Summary and conclusion
Seven residual biomass feedstocks were hydrothermally treated at temperatures ranging from 180 to 270 °C and residence times of 0.5 h, 2 h, and 4 h. The effects of a hydrothermal treatment on properties relevant for combustion were investigated. HTC was found to increase the lower heating value of the treated material with increasing temperature. Residence time was found to have less impact on the fuel properties. At the same time, due to removal of organic material and less removal of inorganic material, the ash content typically increases after an HTC treatment. The inorganic composition of HTC-treated feedstock is fundamentally altered by HTC. K and Cl are removed to a large extent from the fuel by dissolution of salts in the process water, while Si, Ca, and P removal is limited in the solid material. These changes lead to the improvement of fuel properties with respect to corrosion tendency, ash melting temperatures, and fine particle emissions. On the other hand, the fuel N content increases after the treatment leading to a potential higher risk of NOx emissions during its combustion. The results indicate that HTC is suited well for improving the fuel quality of waste streams originating from lignocellulosic feedstock like digestate, empty fruit bunches, lawn cuttings, olive pomace, spruce bark, and wheat straw. Contrary, judging by the indicators used in this study, the fuel quality of sewage sludge could not significantly be improved by HTC. Nonetheless, apart from changing fuel properties related to challenges in biomass combustion, which has been described thoroughly in this work, HTC also offers other beneficial effects like improving storage behavior and dewatering and drying of biofuels. For a holistic assessment of HTC for fuel upgrading, future research that also quantifies the fuel improvements concerning these properties as well as investigation on fuel pricing and logistics is needed.
References
Scarlat N, Dallemand J-F, Monforti-Ferrario F, Nita V (2015) The role of biomass and bioenergy in a future bioeconomy: policies and facts. Environ Develop 15:3–34. https://doi.org/10.1016/j.envdev.2015.03.006
Lago C, Herrera I, Caldés N et al. (2019) Nexus bioenergy–bioeconomy. In: The Role of Bioenergy in the Bioeconomy. Elsevier, pp 3–24
Jenkins BM, Baxter LL, Miles TR (1998) Combustion properties of biomass. Fuel Process Technol 54(1–3):17–46. https://doi.org/10.1016/S0378-3820(97)00059-3
Miles TR, Baxter LL, Bryers RW et al (1996) Boiler deposits from firing biomass fuels. Biomass Bioenergy 10(2–3):125–138. https://doi.org/10.1016/0961-9534(95)00067-4
Näzelius I-L, Boström D, Rebbling A, Boman C, Öhman M (2017) Fuel indices for estimation of slagging of phosphorus-poor biomass in fixed bed combustion. Energy Fuel 31(1):904–915. https://doi.org/10.1021/acs.energyfuels.6b02563
Sommersacher P, Brunner T, Obernberger I (2011) Fuel indexes: a novel method for the evaluation of relevant combustion properties of new biomass fuels. Energy Fuel 26(1):380–390. https://doi.org/10.1021/ef201282y
Nielsen HP, Frandsen FJ, Dam-Johansen K (1999) Lab-scale investigations of high-temperature corrosion phenomena in straw-fired boilers. Energy Fuel 13(6):1114–1121. https://doi.org/10.1021/ef990001g
Aho M, Vainikka P, Taipale R, Yrjas P (2008) Effective new chemicals to prevent corrosion due to chlorine in power plant superheaters. Fuel 87(6):647–654. https://doi.org/10.1016/j.fuel.2007.05.033
Salmenoja K (2000) Field and laboratory studies on chlorine-induced superheater corrosion in boilers fired with biofuels. Dissertation, Abo Akademi University
Johansson LS, Tullin C, Leckner B, Sjövall P (2003) Particle emissions from biomass combustion in small combustors. Biomass Bioenergy 25(4):435–446. https://doi.org/10.1016/S0961-9534(03)00036-9
Williams A, Jones JM, Ma L, Pourkashanian M (2012) Pollutants from the combustion of solid biomass fuels. Prog Energy Combust Sci 38(2):113–137. https://doi.org/10.1016/j.pecs.2011.10.001
Jöller M, Brunner T, Obernberger I (2007) Modeling of aerosol formation during biomass combustion for various furnace and boiler types. Fuel Process Technol 88(11–12):1136–1147. https://doi.org/10.1016/j.fuproc.2007.06.013
Glarborg P (2003) Fuel nitrogen conversion in solid fuel fired systems. Prog Energy Combust Sci 29(2):89–113. https://doi.org/10.1016/S0360-1285(02)00031-X
Houshfar E, Løvås T, Skreiberg Ø (2012) Experimental investigation on NOx reduction by primary measures in biomass combustion: straw, peat, sewage sludge, Forest residues and wood pellets. Energies 5(2):270–290. https://doi.org/10.3390/en5020270
Houshfar E, Skreiberg Ø, Todorović D, Skreiberg A, Løvås T, Jovović A, Sørum L (2012) NOx emission reduction by staged combustion in grate combustion of biomass fuels and fuel mixtures. Fuel 98:29–40. https://doi.org/10.1016/j.fuel.2012.03.044
Peterson AA, Vogel F, Lachance RP et al (2008) Thermochemical biofuel production in hydrothermal media: a review of sub- and supercritical water technologies. Energy Environ Sci 1(1):32. https://doi.org/10.1039/b810100k
Funke A, Ziegler F (2010) Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod Biorefin 4(2):160–177. https://doi.org/10.1002/bbb.198
Hoekman SK, Broch A, Robbins C (2011) Hydrothermal carbonization (HTC) of Lignocellulosic biomass. Energy Fuel 25(4):1802–1810. https://doi.org/10.1021/ef101745n
Libra JA, Ro KS, Kammann C, Funke A, Berge ND, Neubauer Y, Titirici MM, Fühner C, Bens O, Kern J, Emmerich KH (2011) Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2(1):71–106. https://doi.org/10.4155/bfs.10.81
Kambo HS, Dutta A (2014) Strength, storage, and combustion characteristics of densified lignocellulosic biomass produced via torrefaction and hydrothermal carbonization. Appl Energy 135:182–191. https://doi.org/10.1016/j.apenergy.2014.08.094
Kambo HS, Minaret J, Dutta A (2018) Process water from the hydrothermal carbonization of biomass: a waste or a valuable product? Waste Biomass Valor 9(7):1181–1189. https://doi.org/10.1007/s12649-017-9914-0
Kühni M, Wanner R, Baier U et al. (2015) Treatment of process water of hydrothermal carbonised sewage sludge before discharging in a waste water treatment plant 156: 1004–1011
Wirth B, Reza T, Mumme J (2015) Influence of digestion temperature and organic loading rate on the continuous anaerobic treatment of process liquor from hydrothermal carbonization of sewage sludge. Bioresour Technol 198:215–222. https://doi.org/10.1016/j.biortech.2015.09.022
Stemann J, Putschew A, Ziegler F (2013) Hydrothermal carbonization: process water characterization and effects of water recirculation. Bioresour Technol 143:139–146. https://doi.org/10.1016/j.biortech.2013.05.098
Bobleter O (1994) Hydrothermal degradation of polymers derived from plants. Prog Polym Sci 19(5):797–841. https://doi.org/10.1016/0079-6700(94)90033-7
Jin F, Coronella CJ, Lynam JG et al (eds) (2014) Application of hydrothermal reactions to biomass conversion // hydrothermal carbonization of lignocellulosic biomass. Green chemistry and sustainable technology, vol 15. Springer, Heidelberg
Bryers RW (1996) Fireside slagging, fouling, and high-temperature corrosion of heat-transfer surface due to impurities in steam-raising fuels. Prog Energy Combust Sci 22(1):29–120. https://doi.org/10.1016/0360-1285(95)00012-7
Zevenhoven-Onderwater M (2001) Ash-forming matter in biomass fuels. Dissertation. Abo Akademi University, Åbo
Turn SQ, Kinoshita CM, Ishimura DM (1997) Removal of inorganic constituents of biomass feedstocks by mechanical dewatering and leaching. Biomass Bioenergy 12(4):241–252. https://doi.org/10.1016/S0961-9534(97)00005-6
Reza MT, Lynam JG, Uddin MH, Coronella CJ (2013) Hydrothermal carbonization: fate of inorganics. Biomass Bioenergy 49:86–94. https://doi.org/10.1016/j.biombioe.2012.12.004
Smith AM, Singh S, Ross AB (2016) Fate of inorganic material during hydrothermal carbonisation of biomass: influence of feedstock on combustion behaviour of hydrochar. Fuel 169:135–145. https://doi.org/10.1016/j.fuel.2015.12.006
Smith AM, Ross AB (2019) The influence of residence time during hydrothermal carbonisation of miscanthus on bio-coal combustion chemistry. Energies 12(3):523. https://doi.org/10.3390/en12030523
Ulbrich M, Preßl D, Fendt S, Gaderer M, Spliethoff H (2017) Impact of HTC reaction conditions on the hydrochar properties and CO2 gasification properties of spent grains. Fuel Process Technol 167:663–669. https://doi.org/10.1016/j.fuproc.2017.08.010
Vassilev SV, Baxter D, Andersen LK, Vassileva CG, Morgan TJ (2012) An overview of the organic and inorganic phase composition of biomass. Fuel 94:1–33. https://doi.org/10.1016/j.fuel.2011.09.030
Jones RGW, Lunt OR (1967) The function of calcium in plants. Bot Rev 33(4):407–426. https://doi.org/10.1007/BF02858743
Currie HA, Perry CC (2007) Silica in plants: biological, biochemical and chemical studies. Ann Bot 100(7):1383–1389. https://doi.org/10.1093/aob/mcm247
Miles TR, Miles TR, Jr., Baxter LL et al. (1995) Alkali deposits found in biomass power plants: a preliminary investigation of their extent and nature. Volume 1
Engvild KC (1986) Chlorine-containing natural compounds in higher plants. Phytochemistry 25(4):781–791. https://doi.org/10.1016/0031-9422(86)80002-4
Björkman E, Strömberg B (1997) Release of chlorine from biomass at pyrolysis and gasification conditions 1. Energy Fuel 11(5):1026–1032. https://doi.org/10.1021/ef970031o
Kruse A, Koch F, Stelzl K, Wüst D, Zeller M (2016) Fate of nitrogen during hydrothermal carbonization. Energy Fuel 30(10):8037–8042. https://doi.org/10.1021/acs.energyfuels.6b01312
Williams A, Jones JM, Ma L, Pourkashanian M (2012) Pollutants from the combustion of solid biomass fuels. Prog Energy Combust Sci 38(2):113–137. https://doi.org/10.1016/j.pecs.2011.10.001
Acknowledgments
I would like to offer my special thanks to Maximilian Lantzberg and Moritz Böhme for their contribution to this work by conducting experiments.
Availability of data and material
The supplementary data includes the original dataset discussed in this work.
Funding
Open Access funding provided by Projekt DEAL. This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 727616.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 65 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hansen, L.J., Fendt, S. & Spliethoff, H. Impact of hydrothermal carbonization on combustion properties of residual biomass. Biomass Conv. Bioref. 12, 2541–2552 (2022). https://doi.org/10.1007/s13399-020-00777-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00777-z