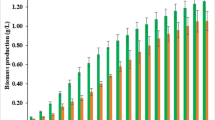
Abstract
Chlorella spp. have been successfully cultured at commercial scale and show potential for the production of advanced biofuels. A current challenge in the downstream processing of Chlorella spp. is the sequential fractionation of whole biomass in different macromolecules such as lipids and carbohydrates. Herein, the biorefinery concept was used to evaluate the best sequence of extraction of carbohydrates and lipids of C. vulgaris. It is also important to develop biorefinery processes that produce green chemicals like ethanol because large volumes of solvents are required for lipid extraction. First, the extraction conditions were optimized to achieve maximum yields of carbohydrates and lipids separately. The extraction of carbohydrates was less complex than the multistage extraction of lipids from C. vulgaris. The use of ethanol and hexane mixture resulted in a lower extraction of lipids from C. vulgaris, even after 4 stages of extraction, and the final lipid content corresponded to only 65% of the lipids extracted with standard methods. Moreover, the multistage extraction of lipids did not improve the subsequent extraction of carbohydrates (lipids-carbohydrates route). However, the dilute sulfuric acid hydrolysis drastically improved the subsequent multistage extraction of lipids (carbohydrates-lipids route), and the lipid yield using this route was twice higher in comparison with the lipids-carbohydrates route. The hydrolysates obtained using the routes carbohydrates-lipids and lipids-carbohydrates showed levels of 2.6 ± 0.2 and 2.7 ± 0.1 g L−1 of total neutral carbohydrates, respectively. The different routes did not exert a great effect on the levels of inhibitors.



Similar content being viewed by others
References
Liu J, Hu Q (2013) Chlorella: industrial production of cell mass and chemicals. In: Handbook of microalgal culture. Wiley, Oxford, pp 327–338
Benemann J (2013) Microalgae for biofuels and animal feeds. Energies 6:5869–5886. https://doi.org/10.3390/en6115869
Gayathri S, Rajasree Radhika SR, Suman TY, Aranganathan L (2018) Ultrasound-assisted microextraction of β, ε-carotene-3, 3′-diol (lutein) from marine microalgae Chlorella salina: effect of different extraction parameters. Biomass Convers Biorefinery 8:791–797. https://doi.org/10.1007/s13399-018-0331-9
Sarayloo E, Simsek S, Unlu YS, Cevahir G, Erkey C, Kavakli IH (2018) Enhancement of the lipid productivity and fatty acid methyl ester profile of Chlorella vulgaris by two rounds of mutagenesis. Bioresour Technol 250:764–769. https://doi.org/10.1016/j.biortech.2017.11.105
Takeshita T, Ota S, Yamazaki T et al (2014) Starch and lipid accumulation in eight strains of six Chlorella species under comparatively high light intensity and aeration culture conditions. Bioresour Technol 158:127–134. https://doi.org/10.1016/j.biortech.2014.01.135
Weinberg J, Kaltschmitt M, Wilhelm C (2012) Analysis of greenhouse gas emissions from microalgae-based biofuels. Biomass Convers Biorefinery 2:179–194. https://doi.org/10.1007/s13399-012-0044-4
Dahlin LR, Van Wychen S, Gerken HG et al (2018) Down-selection and outdoor evaluation of novel, halotolerant algal strains for winter cultivation. Front Plant Sci 9:1–10. https://doi.org/10.3389/fpls.2018.01513
He Q, Yang H, Hu C (2016) Culture modes and financial evaluation of two oleaginous microalgae for biodiesel production in desert area with open raceway pond. Bioresour Technol 218:571–579. https://doi.org/10.1016/j.biortech.2016.06.137
Feng P, Deng Z, Hu Z, Fan L (2011) Lipid accumulation and growth of Chlorella zofingiensis in flat plate photobioreactors outdoors. Bioresour Technol 102:10577–10584. https://doi.org/10.1016/j.biortech.2011.08.109
Guccione A, Biondi N, Sampietro G, Rodolfi L, Bassi N, Tredici MR (2014) Chlorella for protein and biofuels: from strain selection to outdoor cultivation in a green wall panel photobioreactor. Biotechnol Biofuels 7:84. https://doi.org/10.1186/1754-6834-7-84
Chew KW, Yap JY, Show PL et al (2017) Microalgae biorefinery: high value products perspectives. Bioresour Technol 229:53–62. https://doi.org/10.1016/j.biortech.2017.01.006
Laurens LML, Nagle N, Davis R et al (2015) Acid-catalyzed algal biomass pretreatment for integrated lipid and carbohydrate-based biofuels production. Green Chem 17:1145–1158. https://doi.org/10.1039/C4GC01612B
Duongbia N, Chaiwongsar S, Chaichana C, Chaiklangmuang S (2019) Acidic hydrolysis performance and hydrolyzed lipid characterizations of wet Spirulina platensis. Biomass Convers Biorefinery 9:305–319. https://doi.org/10.1007/s13399-018-0350-6
Lee OK, Oh Y-K, Lee EY (2015) Bioethanol production from carbohydrate-enriched residual biomass obtained after lipid extraction of Chlorella sp. KR-1. Bioresour Technol 196:22–27. https://doi.org/10.1016/j.biortech.2015.07.040
Htet AN, Noguchi M, Ninomiya K, Tsuge Y, Kuroda K, Kajita S, Masai E, Katayama Y, Shikinaka K, Otsuka Y, Nakamura M, Honda R, Takahashi K (2018) Application of microalgae hydrolysate as a fermentation medium for microbial production of 2-pyrone 4,6-dicarboxylic acid. J Biosci Bioeng 125:717–722. https://doi.org/10.1016/j.jbiosc.2017.12.026
Kumar SPJ, Kumar GV, Dash A et al (2017) Sustainable green solvents and techniques for lipid extraction from microalgae: a review. Algal Res 21:138–147. https://doi.org/10.1016/j.algal.2016.11.014
Lu W, Wang Z, Yuan Z (2015) Characteristics of lipid extraction from Chlorella sp. cultivated in outdoor raceway ponds with mixture of ethyl acetate and ethanol for biodiesel production. Bioresour Technol 191:433–437. https://doi.org/10.1016/j.biortech.2015.02.069
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv 30:709–732. https://doi.org/10.1016/j.biotechadv.2012.01.001
Borowitzka MA, Moheimani NR (2013) Algae for biofuels and energy. Springer, Dordrecht
Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S, Lee YC (2005) Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal Biochem 339:69–72. https://doi.org/10.1016/j.ab.2004.12.001
Fernie AR, Roscher A, Ratcliffe RG, Kruger NJ (2001) Fructose 2,6-bisphosphate activates pyrophosphate: fructose-6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta 212:250–263. https://doi.org/10.1007/s004250000386
Van Wychen S, Laurens LML (2016) Determination of total carbohydrates in algal biomass: laboratory analytical procedure (LAP). Golden, CO (United States)
Souza MF, Pereira DS, Freitas SP et al (2017) Neutral sugars determination in Chlorella: use of a one-step dilute sulfuric acid hydrolysis with reduced sample size followed by HPAEC analysis. Algal Res 24:130–137. https://doi.org/10.1016/j.algal.2017.03.019
Meijer EA, Wijffels RH (1998) Development of a fast, reproducible and effective method for the extraction and quantification of proteins of micro-algae. Biotechnol Tech 12:353–358. https://doi.org/10.1023/A:1008814128995
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
International Dairy Federation (1987) Cheese and processed cheese products: determination of fat content - gravimetric method - reference method. Brussels
Griffiths MJ, Garcin C, van Hille RP, Harrison STL (2011) Interference by pigment in the estimation of microalgal biomass concentration by optical density. J Microbiol Methods 85:119–123. https://doi.org/10.1016/j.mimet.2011.02.005
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313. https://doi.org/10.1016/S0176-1617(11)81192-2
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Ramluckan K, Moodley KG, Bux F (2014) An evaluation of the efficacy of using selected solvents for the extraction of lipids from algal biomass by the Soxhlet extraction method. Fuel 116:103–108. https://doi.org/10.1016/j.fuel.2013.07.118
Ichihara K, Fukubayashi Y (2010) Preparation of fatty acid methyl esters for gas-liquid chromatography. J Lipid Res 51:635–640. https://doi.org/10.1194/jlr.D001065
Zhang C, Runge T (2012) Fractionating pentosans and hexosans in hybrid poplar. Ind Eng Chem Res 51:133–139. https://doi.org/10.1021/ie201183d
Yao L, Gerde JA, Lee SL, Wang T, Harrata KA (2015) Microalgae lipid characterization. J Agric Food Chem 63:1773–1787. https://doi.org/10.1021/jf5050603
Illman AM, Scragg AH, Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzym Microb Technol 27:631–635. https://doi.org/10.1016/S0141-0229(00)00266-0
Ördög V, Stirk WA, Bálint P et al (2012) Changes in lipid, protein and pigment concentrations in nitrogen-stressed Chlorella minutissima cultures. J Appl Phycol 24:907–914. https://doi.org/10.1007/s10811-011-9711-2
Templeton DW, Quinn M, Van Wychen S et al (2012) Separation and quantification of microalgal carbohydrates. J Chromatogr A 1270:225–234. https://doi.org/10.1016/j.chroma.2012.10.034
Santos VC, Bragança CRS, Passos FJV, Passos FML (2013) Kinetics of growth and ethanol formation from a mix of glucose/xylose substrate by Kluyveromyces marxianus UFV-3. Antonie Van Leeuwenhoek 103:153–161. https://doi.org/10.1007/s10482-012-9794-z
Demeke MM, Dietz H, Li Y, Foulquié-Moreno MR, Mutturi S, Deprez S, den Abt T, Bonini BM, Liden G, Dumortier F, Verplaetse A, Boles E, Thevelein JM (2013) Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulose hydrolysates using metabolic and evolutionary engineering. Biotechnol Biofuels 6:89. https://doi.org/10.1186/1754-6834-6-89
Mu J, Li S, Chen D et al (2015) Enhanced biomass and oil production from sugarcane bagasse hydrolysate (SBH) by heterotrophic oleaginous microalga Chlorella protothecoides. Bioresour Technol 185:99–105. https://doi.org/10.1016/j.biortech.2015.02.082
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507. https://doi.org/10.1016/j.jbiotec.2006.05.002
Cheng D, Li D, Yuan Y, Zhou L, Li X, Wu T, Wang L, Zhao Q, Wei W, Sun Y (2017) Improving carbohydrate and starch accumulation in Chlorella sp. AE10 by a novel two-stage process with cell dilution. Biotechnol Biofuels 10:75. https://doi.org/10.1186/s13068-017-0753-9
Safi C, Zebib B, Merah O et al (2014) Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew Sust Energ Rev 35:265–278. https://doi.org/10.1016/j.rser.2014.04.007
Rodrigues MA, Bon EPS (2011) Evaluation of Chlorella (Chlorophyta) as source of fermentable sugars via cell wall enzymatic hydrolysis. Enzyme Res 2011:1–5. https://doi.org/10.4061/2011/405603
Nanda S, Mohammad J, Reddy SN et al (2014) Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Convers Biorefinery 4:157–191. https://doi.org/10.1007/s13399-013-0097-z
Hernández D, Riaño B, Coca M, García-González MC (2015) Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem Eng J 262:939–945. https://doi.org/10.1016/j.cej.2014.10.049
Show K-Y, Lee D-J, Tay J-H, Lee TM, Chang JS (2015) Microalgal drying and cell disruption – recent advances. Bioresour Technol 184:258–266. https://doi.org/10.1016/j.biortech.2014.10.139
Park C, Lee JH, Yang X, Yoo HY, Lee JH, Lee SK, Kim SW (2016) Enhancement of hydrolysis of Chlorella vulgaris by hydrochloric acid. Bioprocess Biosyst Eng 39:1015–1021. https://doi.org/10.1007/s00449-016-1570-4
Modenbach AA, Nokes SE (2013) Enzymatic hydrolysis of biomass at high-solids loadings - a review. Biomass Bioenergy 56:526–544. https://doi.org/10.1016/j.biombioe.2013.05.031 Review
Zhou S, Runge TM (2014) Validation of lignocellulosic biomass carbohydrates determination via acid hydrolysis. Carbohydr Polym 112:179–185. https://doi.org/10.1016/j.carbpol.2014.05.088
Nguyen MT, Choi SP, Lee J et al (2009) Hydrothermal acid pretreatment of Chlamydomonas reinhardtii biomass for ethanol production. J Microbiol Biotechnol 19:161–166. https://doi.org/10.4014/jmb.0810.578
Miranda JR, Passarinho PC, Gouveia L (2012) Pre-treatment optimization of Scenedesmus obliquus microalga for bioethanol production. Bioresour Technol 104:342–348. https://doi.org/10.1016/j.biortech.2011.10.059
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energy 37:19–27. https://doi.org/10.1016/j.renene.2011.06.045
Peña-Castro JM, Moral S, Núñez-López L et al (2017) Biotechnological strategies to improve plant biomass quality for bioethanol production. Biomed Res Int 2017:1–10. https://doi.org/10.1155/2017/7824076
Soares J, Demeke MM, Van de Velde M et al (2017) Fed-batch production of green coconut hydrolysates for high-gravity second-generation bioethanol fermentation with cellulosic yeast. Bioresour Technol 244:234–242. https://doi.org/10.1016/j.biortech.2017.07.140
Capello C, Fischer U, Hungerbühler K (2007) What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem 9:927. https://doi.org/10.1039/b617536h
Du Y, Schuur B, Brilman DWF (2017) Maximizing lipid yield in Neochloris oleoabundans algae extraction by stressing and using multiple extraction stages with N-ethylbutylamine as switchable solvent. Ind Eng Chem Res 56:8073–8080. https://doi.org/10.1021/acs.iecr.7b01032
Olmstead ILD, Kentish SE, Scales PJ, Martin GJO (2013) Low solvent, low temperature method for extracting biodiesel lipids from concentrated microalgal biomass. Bioresour Technol 148:615–619. https://doi.org/10.1016/j.biortech.2013.09.022
Li Y, Ghasemi Naghdi F, Garg S, Adarme-Vega TC, Thurecht KJ, Ghafor WA, Tannock S, Schenk PM (2014) A comparative study: the impact of different lipid extraction methods on current microalgal lipid research. Microb Cell Factories 13:14. https://doi.org/10.1186/1475-2859-13-14
Montañés F, Olano A, Ibáñez E, Fornari T (2007) Modeling solubilities of sugars in alcohols based on original experimental data. AICHE J 53:2411–2418. https://doi.org/10.1002/aic.11258
Sakarika M, Kornaros M (2017) Kinetics of growth and lipids accumulation in Chlorella vulgaris during batch heterotrophic cultivation: effect of different nutrient limitation strategies. Bioresour Technol 243:356–365. https://doi.org/10.1016/j.biortech.2017.06.110
Almeida JR, Modig T, Petersson A et al (2007) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol 82:340–349. https://doi.org/10.1002/jctb.1676
Funding
This study received research funding from Petróleo Brasileiro S/A (Petrobras grant 4600545175) and was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil (CAPES, Finance Code 001), and by the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq, grant 307147/2015-0). JS was supported by a fellowship from CNPq (grant 155994/2018-2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martins, L.B., Soares, J., da Silveira, W.B. et al. Dilute sulfuric acid hydrolysis of Chlorella vulgaris biomass improves the multistage liquid-liquid extraction of lipids. Biomass Conv. Bioref. 11, 2485–2497 (2021). https://doi.org/10.1007/s13399-020-00661-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00661-w