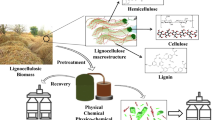
Abstract
The bioconversion of lignocellulosic biomass to second generation (2G) sugars is crucial for the production of biofuels and commodity chemicals. However, due to its recalcitrant nature, pretreatment is an essential step to increase the accessibility of cellulolytic enzymes to hemicellulose and cellulose to obtain 2G sugars. In this study, sugarcane bagasse (SCB) was pretreated by dilute nitric acid (1% w/v), dilute sodium hydroxide (1% w/v) and sequential nitric acid-sodium hydroxide. The pretreated material was then enzymatically hydrolysed (5 and 10% total solids; TS) by cellulase (Cellic Ctec 2, Novozyme, Curitiba, Brazil). Sequential acid–base pretreated bagasse (cellulosic pulp) led the removal of lignin (70.63%) and hemicellulose (100%) and retained 92.33% cellulose. Enzymatic hydrolysis of sequential acid–base pretreated bagasse (5% TS) showed hydrolysis yield of 75.68% (glucose released), followed by sodium hydroxide pretreated with glucose (68.76%) and xylose (73.26%) and nitric acid pretreated with glucose (31.49%) and xylose (31.49%), respectively. Enzymatic hydrolysis of sequential acid–base pretreated (10% TS) showed hydrolysis yield of 66.20% (glucose), followed by glucose (63.02%) and xylose (60.14%) from sodium hydroxide pretreatment and finally glucose (28.71%) and xylose (23.56%) from dilute nitric acid pretreatment. Therefore, the cellulosic material showed high hydrolysis efficiency after enzymatic saccharification, proving that sequential removal of hemicellulose and lignin from the biomass enables high accessibility of cellulases to the substrate, eventually yielding high amount of 2G sugars.

Graphical abstract




Similar content being viewed by others
References
Chandel AK, Garlapati VK, Singh AK, Antunes FAF, Silva SS (2018) The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour Technol 264:370–381
Sustainable Degradation of Lignocellulosic Biomass - Techniques, Applications and Commercialization (2013). ISBN: 978-953-51-1119-1. Editors: Chandel AK and Silva SS, Publisher: InTech Open book Publisher, Rijeka, Croatia.
Valdivia M, Galan JL, Laffarga J, Ramos JL (2016) Biofuels 2020: Biorefineries based on lignocellulosic materials. Microb Biotechnol 9(5):585–594
Canilha L, Chandel AK, Suzane dos Santos Milessi T, Antunes FAF, Luiz da Costa Freitas, W, das Graças Almeida Felipe M, da Silva SS (2012) Bioconversion of sugarcane biomass into ethanol: an overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification, and ethanol fermentation. Biomed Res Int, 2012
Bharathiraja S, Suriya J, Krishnan M, Manivasagan P, Kim SK (2017) Production of enzymes from agricultural wastes and their potential industrial applications. In Advances in Food and Nutrition Research (Vol. 80, pp. 125-148). Academic Press
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioproc 4(1):7
Zhang R, Lu X, Sun Y, Wang X, Zhang S (2011) Modelling and optimization of dilute nitric acid hydrolysis on corn stover. J Chem Technol Biotechnol 86(2):306–314
Sassner P, Mårtensson CG, Galbe M, Zacchi G (2008) Steam pretreatment of H2SO4-impregnated Salix for the production of bioethanol. Bioresour Technol 99(1):137–145
Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuel Bioprod Bioref 2(1):26–40
Hendriks ATWM, Zeeman G (2009) Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour Technol 100(1):10–18
Kim I, Lee B, Park JY, Choi SA, Han JI (2014) Effect of nitric acid on pretreatment and fermentation for enhancing ethanol production of rice straw. Carbohydr Polym 99:563–567
Rodrıguez-Chong A, Ramírez JA, Garrote G, Vázquez M (2004) Hydrolysis of sugar cane bagasse using nitric acid: a kinetic assessment. J Food Eng 61(2):143–152
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29(6):675–685
Singh R, Shukla A, Tiwari S, Srivastava M (2014) A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew Sust Energ Rev 32:713–728
Philippini RR, Martiniano SE, Chandel AK, de Carvalho W, da Silva SS (2017). Pretreatment of sugarcane bagasse from cane hybrids: effects on chemical composition and 2G sugars recovery. Waste Biomass Valor 1-10.
Chandel AK, Antunes FA, Anjos V, Bell MJ, Rodrigues LN, Polikarpov I, da Silva SS (2014) Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid–base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol Biofuels 7(1):63
Shah SSM, Luthfi AAI, Low KO, Harun S, Manaf SFA, Illias RM, Jahim JM (2019) Preparation of kenaf stem hemicellulosic hydrolysate and its fermentability in microbial production of xylitol by Escherichia coli BL21. Sci Rep 9(1):4080
Silva VF, Arruda PV, Felipe MG, Gonçalves AR, Rocha GJ (2011) Fermentation of cellulosic hydrolysates obtained by enzymatic saccharification of sugarcane bagasse pretreated by hydrothermal processing. J Ind Microbiol Biotechnol 38(7):809–817
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D, (2008a) Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure, NREL/TP-510-42618.
Sluiter A, Ruiz R, Scarlata C, Sluiter J, Templeton D (2008b) Determination of extractives in biomass. Laboratory Analytical Procedure, NREL/TP-510-42619
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Canilha L, Santos VT, Rocha GJ, e Silva JBA, Giulietti M, Silva SS et al (2011) A study on the pretreatment of a sugarcane bagasse sample with dilute sulfuric acid. J Ind Microbiol Biotechnol 38(9):1467–1475
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16(3):144–158
Chandel AK, da Silva SS, Carvalho W, Singh OV (2012) Sugarcane bagasse and leaves: foreseeable biomass of biofuel and bio-products. J Chem Technol Biotechnol 87(1):11–20
Rocha GDM, Gonçalves AR, Oliveira BR, Olivares EG, Rossell CEV (2012) Steam explosion pretreatment reproduction and alkaline delignification reactions performed on a pilot scale with sugarcane bagasse for bioethanol production. Ind Crop Prod 35(1):274–279
Pereira PHF, Voorwald HCJ, Cioffi MOH, Mullinari DR, Da Luz SM, Da Silva MLCP (2011) Sugarcane bagasse pulping and bleaching: Thermal and chemical characterization. BioResources 6(3):2471–2482
Chen WH, Ye SC, Sheen HK (2012) Hydrolysis characteristics of sugarcane bagasse pretreated by dilute acid solution in a microwave irradiation environment. Appl Energy 93:237–244
Binod P, Satyanagalakshmi K, Sindhu R, Janu KU, Sukumaran RK, Pandey A (2012) Short duration microwave assisted pretreatment enhances the enzymatic saccharification and fermentable sugar yield from sugarcane bagasse. Renew Energy 37(1):109–116
Chandel AK, Antunes FA, Silva MB, da Silva SS (2013) Unravelling the structure of sugarcane bagasse after soaking in concentrated aqueous ammonia (SCAA) and ethanol production by Scheffersomyces (Pichia) stipitis. Biotechnology for Biofuels 6(1):102
Rezende CA, de Lima MA, Maziero P, Ribeiro de Azevedo E, Garcia W, Polikarpov I (2011) Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol Biofuels 4(1):54
Giese EC, Pierozzi M, Dussán KJ, Chandel AK, da Silva SS (2013) Enzymatic saccharification of acid–alkali pretreated sugarcane bagasse using commercial enzyme preparations. J Chem Technol Biotechnol 88(7):1266–1272
Martin C, Alriksson B, Sjöde A, Nilvebrant NO, Jönsson LJ (2007) Dilute sulfuric acid pretreatment of agricultural and agro-industrial residues for ethanol production. In Applied Biochemistry and Biotecnology (pp. 339-352). Humana Press
Chandel AK, Gajula C, Konakalla R, Rudravaram R, Pogaku R (2011) Bioconversion of pentose sugars into ethanol: a review and future directions. Biotechnol Mole Biol Rev 6(1):008–020
Li BZ, Balan V, Yuan YJ, Dale BE (2010) Process optimization to convert forage and sweet sorghum bagasse to ethanol based on ammonia fibre expansion (AFEX) pretreatment. Bioresour Technol 101(4):1285–1292
Irfan M, Syed Q, Abbas S, Sher MG, Baig S, Nadeem M (2011) FTIR and SEM analysis of thermo-chemical fractionated sugarcane bagasse. Turk J Biochem 36(4)
Zhao X, Zhang L, Liu D (2012) Biomass recalcitrance. Part I: the chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod Biorefin 6(4):465–482
Acknowledgements
We would like to thank the Ipiranga Agroindustrial-Descalvado/São Paulo, Brazil, for providing the sugarcane bagasse samples. We also thank the Novozyme-Curitiba, Brazil, for providing the cellulase (Cellic Ctec 2) for enzymatic hydrolysis.
Funding
AKC is grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, for the financial assistance through visiting professor and researcher program (Process USP number: 15.1.1118.1.0). SSS and JJA are also grateful with the financial support provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo—BIOEN: 08/57926-4 (FAPESP–BIOEN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ascencio, J.J., Chandel, A.K., Philippini, R.R. et al. Comparative study of cellulosic sugars production from sugarcane bagasse after dilute nitric acid, dilute sodium hydroxide and sequential nitric acid-sodium hydroxide pretreatment. Biomass Conv. Bioref. 10, 813–822 (2020). https://doi.org/10.1007/s13399-019-00547-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00547-6