Abstract
Mixed glia are infiltrated with HIV-1 virus early in the course of infection leading to the development of a persistent viral reservoir in the central nervous system. Modification of the HIV-1 genome using gene editing techniques, including CRISPR/Cas9, has shown great promise towards eliminating HIV-1 viral reservoirs; whether these techniques are capable of removing HIV-1 viral proteins from mixed glia, however, has not been systematically evaluated. Herein, the efficacy of adeno-associated virus 9 (AAV9)-CRISPR/Cas9 gene editing for eliminating HIV-1 messenger RNA (mRNA) from cortical mixed glia was evaluated in vitro and in vivo. In vitro, a within-subjects experimental design was utilized to treat mixed glia isolated from neonatal HIV-1 transgenic (Tg) rats with varying doses (0, 0.9, 1.8, 2.7, 3.6, 4.5, or 5.4 µL corresponding to a physical titer of 0, 4.23 × 109, 8.46 × 109, 1.269 × 1010, 1.692 × 1010, 2.115 × 1010, and 2.538 × 1010 gc/µL) of CRISPR/Cas9 for 72 h. Dose-dependent decreases in the number of HIV-1 mRNA, quantified using an innovative in situ hybridization technique, were observed in a subset (i.e., n = 5 out of 8) of primary mixed glia. In vivo, HIV-1 Tg rats were retro-orbitally inoculated with CRISPR/Cas9 for two weeks, whereby treatment resulted in profound excision (i.e., approximately 53.2%) of HIV-1 mRNA from the medial prefrontal cortex. Given incomplete excision of the HIV-1 viral genome, the clinical relevance of HIV-1 mRNA knockdown for eliminating neurocognitive impairments was evaluated via examination of temporal processing, a putative neurobehavioral mechanism underlying HIV-1-associated neurocognitive disorders (HAND). Indeed, treatment with CRISPR/Cas9 protractedly, albeit not permanently, restored the developmental trajectory of temporal processing. Proof-of-concept studies, therefore, support the susceptibility of mixed glia to gene editing and the potential of CRISPR/Cas9 to serve as a novel therapeutic strategy for HAND, even in the absence of full viral eradication.
Similar content being viewed by others
Introduction
The current treatment regimen for HIV-1 seropositive individuals includes a cocktail of two to four drugs, collectively known as combination antiretroviral therapy (cART), which act to suppress viral replication (e.g., Gulick et al. 1997). cART revolutionized the treatment of HIV-1 and transformed the disease into a chronic, manageable condition, whereby HIV-1 seropositive individuals have life expectancies similar to those of seronegative persons (Marcus et al. 2020). Nevertheless, disease management is challenged by poor adherence to cART, which is associated with viral rebound (e.g., Maina et al. 2020). Furthermore, despite treatment with cART, HIV-1 seropositive individuals are disproportionately afflicted by comorbidities (Marcus et al. 2020; e.g., Liver Disorders: Morales et al. 2022; Cardiovascular Disease: Alonso et al. 2019, Touloumi et al. 2020) and exhibit high rates of neurocognitive impairments (e.g., Wang et al. 2020). In light of these observations, scientists have been on a quest to develop a functional cure (i.e., long-term HIV-1 control allowing patients to cease cART for prolonged periods of time or for life) for HIV-1.
Elimination of HIV-1 viral reservoirs, which are established early in the course of infection (Gantner et al. 2023) and persist despite cART (e.g., Chun et al. 1997; Finzi et al. 1997; Wong et al. 1997), presents a key challenge to the development of a functional cure. A viral reservoir, as defined by Eisele and Siliciano (2012), includes infected cell populations that allow long-term persistence (i.e., years) of replication-competent HIV-1 in HIV-1 seropositive individuals on cART. Although the primary HIV-1 viral reservoir resides in memory CD4+ T cells, there is compelling evidence for the existence of additional viral reservoir sites, including the central nervous system (CNS; for review, Churchill et al. 2016, Wallet et al. 2019). Microglia, in particular, are the resident immune cells of the CNS whose lifespan is approximately 4.2 years (Réu et al. 2017). Further, the migration of HIV-1-infected monocytes across the blood-brain barrier (for review, Williams et al. 2012) results in the infection of microglia evidenced by the presence of replication-competent HIV-1 (i.e., deoxyribonucleic acid (DNA) in both untreated (Thompson et al. 2011) and virally suppressed (Ko et al. 2019) HIV-1 seropositive individuals. The ability of microglia to harbor HIV-1 DNA, in conjunction with their long lifespan, meets the criteria for a viral reservoir based on the definition established by Eisele and Siliciano (2012). Eradication of HIV-1 from the CNS, therefore, is likely to be of fundamental import for the successful implementation of a functional cure strategy.
Three broad strategies have been pursued to completely eradicate the HIV-1 genome, including stem cell transplantation (Hutter et al., 2009; Gupta et al. 2019; Jensen et al. 2023; Hsu et al., 2023), “shock and kill” approaches (e.g., for review, Ait-Ammar et al. 2020), and gene editing (e.g., Hu et al. 2014; Qu et al. 2013). Successful eradication of the HIV-1 genome has been achieved in four individuals, whereby three individuals received a CCR5Δ32 homozygous allogeneic adult stem cell transplant (Hutter et al., 2009; Gupta et al. 2019; Jensen et al. 2023) and one adult was cured via a CCR5Δ32/Δ32 haplo-cord transplant (Hsu et al., 2023). Indeed, the HIV-1 genome was successfully eradicated from all four (i.e., 100%) of the patients receiving stem cell transplantation. Despite the recent success of a combined haploidentical and cord blood transplant, which has the potential to increase donor cell availability, widespread implementation of this approach remains impractical. The “shock and kill” approach utilizes latency-reversing agents (LRA; e.g., Tat-R5M4: Geng et al. 2016) to reactivate HIV-1 transcription, thereby enabling the immune system to kill latently infected cells. Although treatment with LRAs induces reactivation of viral transcription in latently infected cells, the viral reservoir size is not reduced in a clinically meaningful manner (i.e., the HIV-1 genome was successfully eradicated in 0% of the patients; e.g., Rasmussen et al., 2014; Gruell et al. 2022). Further, the CNS presents an additional obstacle for the “shock and kill” approach, as it may induce unintended adverse effects, including harmful neuroinflammation and increased neuronal degradation (Gama et al. 2017). Programmable nuclease-based gene editing utilizing zinc finger (Qu et al. 2013) or clustered, regularly-interspaced, short palindromic repeats (CRISPR)/ CRISPR-associated 9 (Cas9) nucleases (e.g., Hu et al. 2014; Kaminski et al. 2016a; Dash et al. 2019), however, represent a promising strategy to eradicate the HIV-1 genome from infected cells.
CRISPRs, which were first identified in the genome of Escherichia coli (Ishino et al. 1987), are a family of repetitive DNA sequences characterized by direct repeats interspaced by similarly sized non-repetitive elements clustered in at least one loci on the chromosome. Upstream of the CRISPR array are CRISPR-associated (cas) genes that encode proteins with nuclease and helicase domains (e.g., Cas9: Makarova et al. 2002). The Cas9 protein, in particular, contains an HNH and RuvC-like nuclease domain; distinct active sites that are utilized to cleave double-stranded DNA (dsDNA; Jinek et al. 2012; Gasiunas et al. 2012). Fundamentally, co-expression of Cas9 and custom-engineered guide ribonucleic acid (gRNA) molecule(s) induces highly efficient site-specific alterations in the target DNA affording a novel tool for gene editing (Jinek et al. 2012, 2013; Cong et al. 2013; Mali et al. 2013).
Indeed, Cas9-mediated genome editing affords a novel strategy to excise the integrated HIV-1 proviral genome in vitro and in vivo (for review, Bhowmik and Chaubey 2022). The development of single gRNA molecules to target the flanking HIV-1 long terminal repeat (LTR) sequences provided a fundamental proof-of-concept, whereby treatment cleaved viral DNA in vitro and inhibited viral gene expression (Ebina et al. 2013; Hu et al. 2014; Kaminski et al. 2016a; Yin et al. 2016). The potential for InDel generation, and thus an “escape mutant”, with a single gRNA (Wang et al. 2016a, b), however, necessitates a multiplex gRNA configuration; a configuration that also efficaciously eradicates the integrated HIV-1 proviral genome in vitro (e.g., Hu et al. 2014; Kaminski et al. 2016a; Yin et al. 2016). Fundamentally, delivery of the Cas9/gRNA system using an adeno-associated virus (AAV) vector excises the HIV-1 provirus in vivo (Kaminski et al. 2016b; Yin et al. 2017; Dash et al. 2019). The efficacy of Cas9/gRNAs for the excision of constitutively expressed HIV-1 viral proteins from the CNS, however, remains understudied.
Thus, complementary in vitro and in vivo aims were undertaken to address this fundamental knowledge gap using the HIV-1 transgenic (Tg) rat. The HIV-1 Tg rat, originally reported by Reid et al. (2001), expresses HIV-1 viral proteins constitutively throughout development (Peng et al. 2010; Abbondanzo and Chang 2014), whereby HIV-1 messenger RNA (mRNA) is predominantly expressed in microglia in a restricted, region-specific manner (Li et al. 2021). Indeed, the prefrontal cortex (PFC), a brain region involved in higher-order cognitive processes (for review, Fuster 2008), which are disrupted by HIV-1 (Cysique et al. 2004; Garvey et al. 2009; Heaton et al. 2011), exhibits high HIV-1 viral protein expression (Li et al. 2021). First, the utility of the Cas9/gRNA system to excise HIV-1 viral proteins in vitro from primary mixed glia cultured from the PFC was established using a dose-response experimental paradigm.Second, the efficacy of the Cas9/gRNA system for HIV-1 mRNA excision in vivo was evaluated in the medial PFC (mPFC). Third, the clinical relevance of HIV-1 mRNA knockdown for mitigating neurocognitive impairments was examined using a longitudinal evaluation of temporal processing; temporal processing is a potential neurobehavioral mechanism underlying higher-order cognitive processes (McLaurin et al. 2019a). Establishing the susceptibility of HIV-1-infected mixed glia to gene editing via AAV9-CRISPR/Cas9 is fundamental to the widespread implementation and success of the therapeutic approach.
Materials and methods
Experimental design
A schematic of the experimental design for the complementary in vitro and in vivo aims is illustrated in Fig. 1.
Animals
For Experiment #1: In Vitro Excision of HIV-1 mRNA, breeding of Fischer F344/N HIV-1 Tg pups was conducted at the University of South Carolina, whereby a Fischer F344/N control female (Envigo Laboratories Inc., Indianapolis, IN, USA) was paired with an HIV-1 Tg male. Primary mixed glia were isolated from no more than one male and one female HIV-1 Tg rat from each litter to preclude violation of the assumption of independence.
For Experiment #2: In Vivo Excision of HIV-1 mRNA and Experiment #3: Clinical Import of CRISPR/Cas9 Excision In Vivo, male and female HIV-1 Tg and control (Fischer F344/N) animals were procured from Envigo Laboratories during early adolescence. Animals were pair-housed with animals of the same sex for the duration of the study.
During both experiments, HIV-1 Tg and control rats had ad libitum access to rodent food (Pro-Lab Rat, Mouse, Hamster Chow #3000) and water. Animals were housed in AAALAC-accredited facilities with environmental conditions targeted at 21˚C ± 2˚C, 50% ± 10% relative humidity, and a 12-h light:12-h dark cycle with lights on at 0700 h (EST). Guidelines established by the National Institutes of Health in the Guide for the Care and Use of Laboratory Animals were utilized for the maintenance of all animals. The University of South Carolina Institutional Animal Care and Use Committee approved the project protocol under Federal Assurance (#D16-00028).
Experiment #1: In Vitro Excision of HIV-1 mRNA
Primary mixed glial cell cultures
The prefrontal cortex was dissected from male (n = 4) and female (n = 4) neonatal (i.e., Postnatal Day 1–3) HIV-1 Tg rat pups for the isolation of mixed glia. Following dissection, brain tissue was transferred into a 15 mL centrifuge tube with Hank’s balanced salt solution (HBSS) buffered with 10 mM HEPES (GIBCO Life Technologies, Grand Island, NY, USA) and 0.25% Trypsin/EDTA (GIBCO Life Technologies); the centrifuge tube was incubated at 37˚C in a 5% CO2/95% room air-humidified incubator for 20 min. Tissue was dissociated using trituration and transferred into a 75 mm poly-L-lysine coated flask with DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS). DMEM/F12 medium supplemented with 10% FBS was changed the day after isolation and supplemented every three to seven days. Upon reaching at least 90% confluency, mixed glia were reseeded into poly-L-lysine coated 8-well CultureSlides (Corning, Corning, NY, USA) for experimentation. The morphological characteristics of primary mixed glial cultures at 90% confluency support astrocytes and microglia as the predominant cell types, whereby astrocytes were characterized by a monolayer of flat, polygonal cells resembling those shown by Souza et al. (2013). Microglia, which were loosely adhered to confluent astrocytes, were characterized by a round, ameboid-like phenotype, consistent with previous reports (Cristóvão et al. 2010; Caldeira et al. 2014). Cultures were maintained in the incubator (i.e., 37˚C in 5% CO2 and 95% humidity) at all times.
CRISPR/Cas9 plasmid and treatment
A detailed description of the guide RNA (gRNA) design, construction of the CRISPR/Cas9 expression plasmid, and adeno-associated virus 9 (AAV9) vector have been previously published (Hu et al. 2014). In brief, gRNAs with high guide efficiency (i.e., to maximize on-target activities in the HIV-1 genome) and specificity (i.e., to minimize off-target effects in the human genome) were selected based on screening using the Broad Institute gRNA designer tool; screening which yielded two gRNAs targeting the HIV-1 LTR promotor region and the gag gene. A plasmid carrying the SaCas9 endo-nuclease gene under the CMV promoter and gRNAs was packaged into an AAV9 serotype (Vigene Biosciences Inc., Milton Park, Abingdon, UK). An AAV9 delivery vector was selected for the delivery of CRISPR/Cas9 given its robust transduction efficiency into the central nervous system (Foust et al. 2009). Cortical mixed glial cell cultures were treated for 72 h with one of seven doses (i.e., 0, 0.9, 1.8, 2.7, 3.6, 4.5, or 5.4 µL corresponding to a physical titer of 0, 4.23 × 109, 8.46 × 109, 1.269 × 1010, 1.692 × 1010, 2.115 × 1010, and 2.538 × 1010 gc/µL) of AAV9-SaCas9-sgRNA-HIV-LTR1/GagD (4.7 × 1012 gc/mL).
RNAscope in situ hybridization
A highly sensitive and specific RNAscope in situ hybridization technique (Advanced Cell Diagnostics, Inc., Newark, CA, USA) was utilized to detect the presence of HIV-1 mRNA. Seventy-two hours after CRISPR/Cas9 treatment, 8-well CultureSlides were submerged into phosphate-buffered saline (PBS; 1x) and cells were fixed using 10% neutral buffered formalin (Sigma-Aldrich, St. Louis, MO, USA). After washing with PBS, cells were dehydrated using an increasing ethanol (EtOH) gradient (i.e., 50% EtOH for 5 min, 70% EtOH for 5 min, 100% EtOH for 5 min, 100% EtOH for 10 min). Slides were stored at -20˚C until further experimentation.
Upon continuation of the in situ hybridization assay, cells were rehydrated using a decreasing EtOH gradient (i.e., 70% EtOH for 2 min, 50% EtOH for 2 min) followed by PBS (10 min). A hydrophobic barrier was created around each well using the ImmEdge Hydrophobic Barrier PAP Pen (Vector Laboratories, Burlingame, CA, USA) and allowed to dry completely. After being rinsed with PBS, the RNAscope Protease III reagent (Advanced Cell Diagnostics, Inc.) was added to cells in each well and incubated in the HybEZ Humidity Control Tray (Advanced Cell Diagnostics, Inc.). Slides were washed twice by agitating them in PBS.
RNA in situ hybridization was subsequently conducted using the RNAscope Multiplex Fluorescent Assay (Advanced Cell Diagnostics, Inc., Newark, CA, USA). Detailed methodological procedures for the RNAscope Multiplex Fluorescent Assay are presented in Li et al. (2018) with minor modifications. Specifically, cells were hybridized with a specific probe for HIV-1 mRNA (i.e., Probe-V-HIV1-CladeB-vif-vpr-tat-rev-vpu-env-nef-tar, Catalogue Number: 444,061, Advanced Cell Diagnostics, Inc.). Additionally, following all amplification steps, cells were counterstained with DAPI for 30 s prior to mounting (ProLong Gold Antifade, Invitrogen, Carlsbad, CA, USA) and coverslipping.
HIV-1 mRNA quantification
Multiple z-stack images were obtained from high confluency areas, as evidenced by DAPI staining, in each well using a Nikon TE-2000E confocal microscope system controlled by Version 3.81b of Nikon’s EZ-C1 software. Images were captured at 60x using an oil objective with a numerical aperture of 1.4 and z-plane intervals of 0.15 μm. Fluorophore excitation of the HIV-1 mRNA probe and DAPI staining were accomplished using an argon helium neon laser (Emission: 488 nm) and HeNe helium-neon (Emission: 633 nm), respectively.
HIV-1 mRNA exhibited a “discrete dots” (Li et al. 2018) staining pattern affording an opportunity to quantify the number of HIV-1 mRNA. Z-stack images were blinded to prevent experimental bias. The number of HIV-1 mRNA signals was counted from two z-stack images per well based on established selection criteria (i.e., high cell confluency, bright HIV-1 mRNA signal). To preclude violation of the assumption of independence, the number of HIV-1 mRNA signals from the two z-stack images per well was averaged (Denenberg 1984; Wears 2002).
Experiment #2: In Vivo Excision of HIV-1 mRNA
Retro-orbital CRISPR/Cas9 inoculation
Adult male and female HIV-1 Tg animals were randomly assigned to receive retro-orbital injections of either CRISPR/Cas9 (i.e., AAV9-SaCas9-sgRNA-HIV-LTR1/GagD, 4.7 × 1012 gc/mL; n = 4; Male: n = 2, Female: n = 2) or saline (n = 4; Male: n = 2, Female: n = 2). Retro-orbital inoculations were chosen as a less invasive (i.e., relative to stereotaxic surgeries) and translationally relevant (i.e., intravenous delivery) approach. After inhalant anesthesia was induced with sevoflurane, the animal was placed laterally with the injection-eye facing upwards. A 1 cc tuberculin syringe with a 26G needle was slowly inserted into the medial canthus of the eye at a 45˚ angle to the nose. 50 µL (i.e., Physical Titer: 2.35 × 1011 gc/µL) of CRISPR/Cas9 or saline was gently injected into the retro-orbital vessels. Animals were allowed to recover in a heat-regulated warm chamber.
Tissue Preparation
Approximately two weeks after CRISPR/Cas9 inoculation, anesthesia was induced using 5% sevoflurane, and HIV-1 Tg rodents were humanely sacrificed. Within five minutes of sacrifice, the brain was harvested from the skull and frozen in liquid nitrogen for 15-sec. The brain was sliced into coronal sections using a cryostat (30 μm) and transferred onto slides (SuperFrost Plus Slides, Thermo Fisher Scientific, Hampton, NH, USA) for in situ hybridization.
In situ hybridization using RNAscope
HIV-1 mRNA expression in the mPFC was evaluated using a highly specific and sensitive RNA in situ hybridization technique. Minor modifications to the RNA in situ hybridization technique, described in detail by Li et al. (2018), were implemented in the present study. Specifically, the coronal sections including the mPFC (approximately 3.7 mm to 2.2 mm anterior to Bregma; Paxinos and Watson 2014) were hybridized with a probe specific for HIV-1 mRNA (i.e., Probe-V-HIV1-CladeB-vif-vpr-tat-rev-vpu-env-nef-tar, Catalogue Number: 444,061, Advanced Cell Diagnostics, Inc.).
Experiment #3: Clinical Import of CRISPR/Cas9 Excision In Vivo
Retro-orbital injections of CRISPR/Cas9
Upon arrival at the animal vivarium, F344/N control and HIV-1 Tg animals were randomly assigned to receive retro-orbital injections of CRISPR/Cas9 (HIV-1 Tg, n = 8; Male: n = 4, Female: n = 4), the viral vector HM4d (Control, n = 8; Male: n = 4, Female: n = 4), or saline (Control, n = 8; Male: n = 4, Female: n = 4; HIV-1 Tg, n = 8; Male: n = 4, Female: n = 4). Animals were inoculated at postnatal day (PD) 32, 40, and 102. Retro-orbital inoculations were conducted as described in Experiment #2.
Assessments of Temporal Processing
Apparatus
The startle platform (SR-Lab Startle Reflex System, San Diego Instruments, Inc., San Diego, CA) and Plexiglas test cylinder were enclosed within a double-walled isolation cabinet (External Dimensions: 81 × 81 × 116 cm; Industrial Acoustic Company, Inc., Bronx, NY) that provided 30 dB(A) of sound attenuation. In the absence of any stimuli, the ambient sound level within the isolation cabinet was 22 dB(A). Auditory prepulse and startle stimuli were presented using a high-frequency loudspeaker (Model #40-1278B, Radio Shack, Fort Worth, TX) that was mounted 30 cm above the Plexiglas test cylinder. A 22 lx white LED light, which was mounted on the wall of the isolation chamber, was utilized to present visual prepulse stimuli. A deflection of the Plexiglas test cylinder was produced by the animal’s response to the auditory startle stimulus. Response signals were converted into analog signals by a piezoelectric accelerometer integral to the Plexiglas test cylinder, digitized (12-bit A to D), and saved to a hard disk.
Prepulse inhibition of the auditory startle response
Prior to an assessment of temporal processing, animals were habituated using the test procedure previously described (McLaurin et al. 2017a). Temporal processing was subsequently evaluated using a 30-minute cross-modal prepulse inhibition experimental paradigm (described in detail by McLaurin et al. 2017a). In brief, after a 5-minute acclimation period, the startling stimulus was presented for six trials; trials which were separated by a fixed 10-sec intertrial interval. During the subsequent 72 testing trials, a visual or auditory prepulse was presented prior to the auditory startling stimulus at interstimulus intervals (ISIs) of 0, 30, 50, 100, 200, or 4000 msec; the 0 and 4000 msec ISI trials served as control trials to provide a reference auditory startle response (ASR) within the test session. Counterbalancing (i.e., ABBA) was implemented for the presentation of auditory and visual prepulse trials, while a Latin-square experimental design was utilized for the presentation of ISIs within 6-trial blocks. The intertrial interval for testing trials was variable, whereby it ranged from 15 to 25 s. Mean peak ASR amplitude values were collected for subsequent analyses.
Statistical analysis
Statistical analyses, including analysis of variance (ANOVA) and regression techniques, were conducted using SPSS Statistics 28 (IBM Corporation, Somer, NY) and GraphPad Prism 5 (La Jolla, CA), respectively. GraphPad Prism 5 was also used for the creation of figures. An α criterion of p ≤ 0.05 was set for the establishment of statistical significance.
Two approaches were utilized to evaluate the susceptibility of mixed glia to gene editing via CRISPR/Cas9 in vitro. First, statistical analyses were conducted on all primary mixed glia (n = 8). Second, when the overall analysis failed to reveal any statistically significant effects, complementary analyses that included a subset of animals were conducted. Indeed, the number of HIV-1 mRNA from primary mixed glia cultured from a subset of neonatal HIV-1 Tg rats was examined dependent upon which phenomena (i.e., excision at high (5.4 µL (2.538 × 1010 gc/µL); n = 3) or low (1.8 µL (8.46 × 109 gc/µL; n = 2) dose of CRISPR/Cas9) they exhibited. Data were analyzed using a repeated measures ANOVA, whereby CRISPR/Cas9 dose served as a within-subjects factor. Two CRISPR/Cas9 doses were missing from primary mixed glia cultured from one neonatal HIV-1 Tg rat exhibiting significant excision when treated with 5.4 µL (2.538 × 1010 gc/µL) of CRISPR/Cas9; mean imputation was utilized for the repeated-measures ANOVA. Complementary linear regression analyses were also conducted.
The in vivo efficacy of CRISPR/Cas9 was evaluated using a univariate ANOVA, whereby the number of HIV-1 mRNA in the mPFC served as the dependent variable of interest. Treatment served as the between-subjects factor. Further, the clinical relevance of HIV-1 mRNA knockdown for mitigating neurocognitive impairments was evaluated using linear regression analyses, whereby two dependent variables of interest (i.e., Startle Response, Prepulse Inhibition (PPI) were derived from the ISI function (McLaurin et al. 2019b) and examined using a priori planned comparisons. Specifically, startle response was calculated as an average between the 0 and 4000 msec ISI trials, whereas the area of inflection of the ASR amplitude response curve was calculated as an index of PPI (McLaurin et al. 2016). Furthermore, the genotype deficit (i.e., Control vs. HIV-1 Tg Saline) and effect of CRISPR/Cas9 (i.e., Control vs. HIV-1 Tg CRISPR/Cas9) were established using a priori planned comparisons. Given that the viral vector HM4d had no statistically significant effect on either of the dependent variables of interest, the control data presented and analyzed were collapsed across viral infusion.
Results
Experiment #1: In Vitro Excision of HIV-1 mRNA
CRISPR/Cas9 efficaciously excised HIV-1 viral proteins from primary mixed glia in a subset of neonatal HIV-1 Tg rats
Primary mixed glia were cultured from the PFC of HIV-1 Tg rats, which express seven of the nine HIV-1 viral proteins constitutively throughout development (Peng et al. 2010; Abbondanzo and Chang 2014). Cells were treated with one of seven doses of CRISPR/Cas9 (i.e., 0, 0.9, 1.8, 2.7, 3.6, 4.5, or 5.4 µL corresponding to a physical titer of 0, 4.23 × 109, 8.46 × 109, 1.269 × 1010, 1.692 × 1010, 2.115 × 1010, and 2.538 × 1010 gc/µL) for 72 h, after which time the number of HIV-1 mRNA, visualized using RNAscope in situ hybridization, were quantified. Pronounced HIV-1 mRNA expression was observed in cells not treated with CRISPR/Cas9 (i.e., 0 µL; Number of HIV-1 mRNA (Mean ± Standard Error of the Mean): 375.4 ± 40.5) A subset (i.e., n = 5 out of 8) of primary mixed glia isolated from neonatal HIV-1 Tg rats exhibited dose-dependent decreases in HIV-1 mRNA following CRISPR/Cas9 treatment (Fig. 2). Indeed, two phenomena were observed, whereby HIV-1 mRNA was efficaciously excised following treatment with either high (i.e., 5.4 µL (2.538 × 1010 gc/µL); n = 3) or low (i.e., 1.8 µL (8.46 × 109 gc/µL); n = 2) doses of CRISPR/Cas9.
HIV-1 mRNA was efficaciously excised from primary mixed glia in a subset (i.e., n = 5 out of 8) of neonatal HIV-1 Tg rats, whereby two prominent phenomena were observed. A First, pronounced excision of HIV-1 mRNA was observed in mixed glia cultured from three neonatal HIV-1 Tg rat pups following treatment with 5.4 µL (2.538 × 1010 gc/µL) of CRISPR/Cas9. B-C Quantification of the number of HIV-1 mRNA signals revealed dose-dependent excision efficiency that was well-described by a segmental linear regression; observations which were confirmed by expressing the data as a percentage change relative to the respective untreated control. D Second, low doses of CRISPR/Cas9 (i.e., 1.8 µL (8.46 × 109 gc/µL) were sufficient to excise mixed glia cultured from two neonatal HIV-1 Tg rat pups. E Expression of the data as a percentage change relative to the respective untreated control revealed a linear decrease from 0 (0 gc/µL) to 1.8 µL (8.46 × 109 gc/µL)
First, in mixed glia cultured from three neonatal HIV-1 Tg rat pups, pronounced excision of HIV-1 mRNA was observed following treatment with 5.4 µL (2.538 × 1010 gc/µL) of CRISPR/Cas9 (Fig. 2A). The number of HIV-1 mRNA signals was well-described by a segmental linear regression (R2 ≥ 0.90; Fig. 2B), whereby a linear decrease in the number of HIV-1 mRNA signals was observed from 3.6 µL (1.692 × 1010 gc/µL) to 5.4 µL (2.538 × 1010 gc/µL; Main Effect of Dose with a Primary Quadratic Component: F(1,2) = 182.5, p ≤ 0.005, ηp2 = 0.989). Expressing the data as a percentage change relative to the respective untreated control confirmed dose-dependent excision efficiency that was best-fit using a segmental linear regression (R2 ≥ 0.88; Fig. 2C). Indeed, at the 5.4 µL (2.538 × 1010 gc/µL) dose of CRISPR/Cas9, the average excision efficiency of HIV-1 mRNA from primary mixed glia was 57.1% (Range: 46.9–70.5%).
Second, HIV-1 mRNA was efficaciously excised from mixed glia cultured from two neonatal HIV-1 Tg rat pups after treatment with low (i.e., 1.8 µL ((8.46 × 109 gc/µL)) doses of CRISPR/Cas9 (Fig. 2D). From 0 µL (0 gc/µL) to 1.8 µL (8.46 × 109 gc/µL), the number of HIV-1 mRNA signals, expressed as a percentage change relative to the respective untreated control, decreased in a linear manner (Best-Fit Function: First-Order Polynomial, R2 ≥ 0.81; Fig. 2E). The average excision efficiency of HIV-1 mRNA from primary mixed glia at the 1.8 µL dose of CRISPR/Cas9 was 41.9% (Range: 39.7–44.2%).
Experiment #2: In Vivo Excision of HIV-1 mRNA
Retro-orbital inoculation with CRISPR/Cas9 significantly decreases HIV-1 mRNA in the medial prefrontal cortex of HIV-1 Tg rats
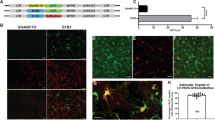
Subsequent in vivo experiments were undertaken to further evaluate the ability of CRISPR/Cas9 to efficaciously excise HIV-1 mRNA from the CNS of HIV-1 Tg rats (Saline: n = 4, Male: n = 2, Female: n = 2; CRISPR/Cas9: n = 4, Male: n = 2, Female: n = 2). CRISPR/Cas9 or saline was retro-orbitally inoculated into the orbital venous plexus, which flows through the superior ophthalmic vein to the cavernous sinus (i.e., part of the brain’s dural venous sinuses; Ngnitewe Massa et al. 2022). Approximately two weeks after retro-orbital inoculation with CRISPR/Cas9 or saline, in situ hybridization techniques were utilized to visualize and quantify the number of HIV-1 mRNA. Profound HIV-1 mRNA expression was observed in HIV-1 Tg animals treated with saline (Fig. 3A–B). HIV-1 mRNA expression was significantly decreased by treatment with CRISPR/Cas9 (Main Effect of Treatment: F(1,6) = 29.3, p ≤ 0.002, ηp2 = 0.830), whereby excision efficiency was approximately 53.2%. Taken together, these proof-of-concept observations in vitro and in vivo support the susceptibility of mixed glia to efficacious gene editing via AAV9-CRISPR/Cas9.
Efficacious excision of HIV-1 mRNA from the central nervous system of HIV-1 transgenic rats was observed in vivo. A Prominent HIV-1 mRNA expression was observed in HIV-1 Tg animals treated with saline; expression levels which were significantly decreased by retro-orbital inoculation with CRISPR/Cas9. B Quantification of the number of HIV-1 mRNA signals confirms significant excision following CRISPR/Cas9 treatment, whereby excision efficiency was approximately 53.2%
Experiment #3: Clinical Import of CRISPR/Cas9 Excision In Vivo
Complete excision of the HIV-1 genome may be unnecessary to enhance neurocognitive function
Given incomplete excision, subsequent in vivo studies were conducted to establish the clinical relevance of HIV-1 mRNA knockdown. For example, the mitigation of HIV-1-associated neurocognitive disorders (HAND), which afflict approximately 50% of HIV-1 seropositive individuals (Wang et al. 2020), has the potential to improve the quality of life for this population. Therefore, following retro-orbital inoculation with CRISPR/Cas9, the viral vector HM4d, or saline, temporal processing, a potential neurobehavioral mechanism underlying HAND (McLaurin et al. 2019a), was evaluated longitudinally using PPI. Two dependent variables of interest (i.e., Startle Response and PPI) were examined using a priori planned comparisons to establish an HIV-1 genotype effect (i.e., Control vs. HIV-1 Tg Saline) and the magnitude of the treatment effect (i.e., Control vs. HIV-1 CRISPR/Cas9). Given that the viral vector HM4d had no statistically significant effect on either of the dependent variables of interest, the control data presented and analyzed were collapsed across viral infusion.
Constitutive expression of the HIV-1 transgene induces pronounced alterations in the development of temporal processing, indexed using startle response (Fig. 4A) and PPI (Fig. 4B). HIV-1 Tg animals treated with saline failed to exhibit any statistically significant development of either startle response or PPI from PD 60 to PD 120, whereby data were well-described by a horizontal line. In sharp contrast, the development of mean startle response and PPI in control animals was well-described by a first-order polynomial (R2 ≥ 0.84) and segmental linear regression (R2 ≥ 0.96), respectively. Regression analyses, therefore, illustrate the prominent temporal processing deficit induced by HIV-1 viral protein exposure.
Even in the absence of complete excision of the HIV-1 viral genome, treatment with CRISPR/Cas9 is clinically relevant. Startle response A, C and prepulse inhibition PPI; B, D were derived from the interstimulus interval function. HIV-1 Tg animals treated with saline exhibited profound deficits in the development of both startle response A and PPI B. Treatment with CRISPR/Cas9, however, partially restored the developmental trajectory of startle response C and PPI D
Retro-orbital inoculation with CRISPR/Cas9, however, protractedly, albeit not permanently, restored the development of temporal processing in HIV-1 Tg animals, evidenced by measures of startle response (Fig. 4C) and PPI (Fig. 4D). From PD 60 to PD 90, the developmental trajectory of startle response and PPI in HIV-1 Tg animals treated with CRISPR/Cas9 resembles that of control animals. Indeed, HIV-1 Tg animals inoculated with CRISPR/Cas9 exhibited a linear increase in startle response through PD 90 followed by a subsequent decrease (Best Fit Function: Segmental Linear Regression, R2 ≥ 0.90). Overlapping 90% confidence intervals between HIV-1 Tg animals treated with CRISPR/Cas9 and control animals support statistically indistinguishable developmental trajectories. With regards to PPI, the linear increase from PD 60 to PD 90 observed in both HIV-1 Tg animals treated with CRISPR/Cas9 and control animals was well-described by a global fit (i.e., First Order Polynomial: R2 ≥ 0.78), whereby no statistically significant differences in the parameters of the function were observed (p ≥ 0.05). Taken together, even in the absence of complete excision, the knockdown of HIV-1 mRNA enhances neurocognitive function for a protracted period of time.
Discussion
Proof-of-concept studies support the susceptibility of mixed glia to gene editing via CRISPR/Cas9, whereby pronounced, albeit incomplete, excision of the HIV-1 viral genome was observed both in vitro and in vivo. In vitro, a subset (i.e., n = 5 out of 8) of primary mixed glia isolated from neonatal HIV-1 Tg rats exhibited dose-dependent decreases in the number of HIV-1 mRNA following CRISPR/Cas9 treatment. Efficacious excision, defined as at least a 35% decrease in the number of HIV-1 mRNA, from primary mixed glia occurred at either the 1.8 µL (8.46 × 109 gc/µL) or 5.4 µL (2.538 × 1010 gc/µL) dose. In vivo, retro-orbital inoculation of CRISPR/Cas9 into the orbital venous plexus of HIV-1 Tg rats resulted in profound excision (i.e., approximately 53.2%) of HIV-1 mRNA from the mPFC. Nevertheless, excision of the complete HIV-1 viral genome may be unnecessary for the mitigation of HAND, as the developmental trajectory of temporal processing was protractedly restored in HIV-1 Tg animals treated with CRISPR/Cas9. Thus, independent of full viral eradication, gene editing via CRISPR/Cas9 may afford a novel therapeutic strategy for HAND.
Significant individual variability in the efficacy of CRISPR/Cas9 in primary mixed glial cultures (i.e., pronounced excision in only a subset n = 5 out of 8 cultures) may be due, at least in part, to the dsDNA repair pathway utilized by cells. Efficacious gene editing using CRISPR/Cas9 enzymes relies upon the cleavage of dsDNA at locations precisely defined by custom-engineered gRNA molecule(s); genome integrity and the viability of the cell, however, are dependent upon proper repair of dsDNA breaks. dsDNA breaks are primarily repaired via one of three processes, including non-homologous end joining (NHEJ), microhomology-mediated end joining (MMEJ), or homology-directed repair (HDR). Although NHEJ and MMEJ are recognized as the main repair mechanisms for dsDNA breaks, these pathways, which act by re-ligating broken DNA ends in the absence of a DNA template from an exogenous donor, are error-prone (for review, Xue and Greene 2021). Indeed, with specific regard to HIV-1, mutations induced by NHEJ processes facilitated viral escape (Wang et al. 2016a, b). In sharp contrast, HDR is the preferred repair mechanism for CRISPR/Cas9-induced dsDNA breaks, as it more accurately repairs the genome via a homologous donor template; upregulation of end joining dsDNA repair mechanisms (i.e., NHEJ, MMEJ) in eukaryotic cells, however, limits the extent to which HDR is utilized (Xue and Greene 2021). Hence, regulating the pathway used to repair dsDNA breaks during genome editing has the potential to enhance the efficacy and precision of CRISPR/Cas9 genome editing.
The utilization of primary mixed glial cultures, rather than purified microglia, to evaluate the efficacy of CRISPR/Cas9 is of fundamental importance. Under homeostatic conditions in vivo, the morphology of microglial cells is characterized by a small cell body and very fine, ramified processes. Purified microglia in culture, however, display an amoeboid-like phenotype (Cristóvão et al. 2010; Caldeira et al. 2014) and express a lysosomal enzyme (i.e., CD68) associated with phagocytic activity during the first ten days in vitro (Cristóvão et al. 2010). Changes to environmental tissue conditions (i.e., in vitro vs. in vivo) also induces pronounced alterations in the transcriptomes and epigenomic features of microglia (Bohlen et al. 2017; Gosselin et al. 2017), whereby gene expression alterations occur in a time-dependent manner (Bohlen et al. 2017). Indeed, serum supplementation, which has historically been utilized to stimulate cellular growth (Puck et al. 1958) and considered fundamental to the viability of cells in culture (Puck et al. 1958), is involved in the perturbation of microglial properties in vitro (Bohlen et al. 2017; Montilla et al. 2020). In light of these findings, serum-free conditions resembling the physiological condition of cerebrospinal fluid have been defined; conditions that also promote microglial survival and a ramified microglial morphology in vitro (Bohlen et al. 2017; Montilla et al. 2020). Nevertheless, results derived from only primary microglial cultures should be interpreted with caution, as in vitro models are not without limitation.
Similarly, the validity of in vivo studies is dependent upon the utilization of a biological system that recapitulates key aspects of the clinical phenotype. Characteristics of rats, including their effective regulation of tat transactivation and the presence of compatible cellular cofactors that permit the production of HIV-1 viral proteins (Yedavalli et al. 2003), renders them an appropriate species for a transgenic HIV-1 biological system. In 2001, Reid et al. (2001) reported the development of a hemizygous HIV-1 Tg rat that contains a non-infectious transgenic construct consisting of a gag-pol deleted (i.e., non-replicative) HIV-1 provirus regulated by the human viral LTR. In total, seven of the nine HIV-1 genes (i.e., env, nef, rev, tat, vif, vpr, and vpu) are expressed constitutively throughout development in the HIV-1 Tg rat (Peng et al. 2010; Abbondanzo and Chang 2014) resembling HIV-1 seropositive individuals on cART. Features of the HIV-1 Tg rat support its utility as a biological system to model neuroHIV (for review, Vigorito et al. 2015) and noninfectious comorbidities (Denaro et al. 2020). Indeed, many of the neuropathological hallmarks of neuroHIV are recapitulated in the HIV-1 Tg rat, including the expression of HIV-1 mRNA in microglia (Clinical: Ko et al. 2019, Li et al. 2023; HIV-1 Tg Rat: Li et al. 2021), profound synaptodendritic injury (Clinical: Moore et al. 2006, Weiss et al. 2021; HIV-1 Tg Roscoe et al. 2014; McLaurin et al. 2018a, 2021), and progressive neurocognitive impairments (Clinical: Heaton et al. 2015, Sacktor et al. 2016, Rubin et al. 2017, Gott et al. 2017; HIV-1 Tg Rat: McLaurin et al. 2016, 2019c). Hence, the HIV-1 Tg rat afforded an in vivo biological system with compelling face validity to further evaluate the susceptibility of mixed glia to gene editing via CRISPR/Cas9.
HIV-1 Tg rats retro-orbitally inoculated with CRISPR/Cas9 exhibited a pronounced decrease in the number of HIV-1 mRNA in the mPFC relative to their saline-inoculated counterparts. Recombinant adeno-associated viral (rAAV) vectors, which are utilized to deliver exogenous DNA to rodents (e.g., CRISPR/Cas9), can be intravenously delivered via the tail vein (Foust et al. 2009; Gray et al. 2011), facial vein (Foust et al. 2009), or retro-orbital venous sinus (Prabhakar et al. 2019; for protocol, Prabhakar et al. 2021; Present Study). During retro-orbital inoculation, rAAV vectors are slowly injected into the orbital venous plexus, which flows through the superior ophthalmic vein to the cavernous sinus (i.e., part of the brain’s dural venous sinuses; Ngnitewe Massa et al. 2022) providing direct access to the CNS. The advantages of retro-orbital, rather than tail vein, injections cannot be understated, as retro-orbital injections are suitable for all ages (e.g., Newborn: Gruntman et al. 2017, Adults: for protocol, Yardeni et al. 2011) and induce a lower stress response (Steel et al. 2008). Indeed, retro-orbital inoculation of HIV-1 Tg rats with CRISPR/Cas9 illustrates the utility of the delivery approach, as well as the susceptibility of mixed glia to gene editing in vivo.
Despite incomplete excision of the HIV-1 viral genome, treatment with CRISPR/Cas9 has profound clinical relevance, as the knockdown of HIV-1 mRNA enhances temporal processing for a protracted period of time. PPI of the ASR, a translational experimental paradigm introduced and popularized by Hoffman and Ison (Hoffman and Searle 1965; Ison and Hammond 1971), is utilized to tap temporal processing, a construct analogous to the speed of information processing in humans. Indeed, HIV-1 seropositive individuals with HAND exhibited profound impairments in PPI, assessed using an eyeblink startle experimental paradigm, relative to their cognitively intact counterparts (Minassian et al. 2013). The HIV-1 Tg rat recapitulates and extends the PPI deficits reported in HIV-1 seropositive individuals with HAND, as temporal processing alterations in the HIV-1 Tg rat occur early in development (McLaurin et al. 2017b), progress across time (Moran et al. 2013; McLaurin et al. 2016, 2018b), and mediate alterations in higher-order cognitive processes (McLaurin et al. 2019a). The ability of PPI to partially mediate the relationship between HIV-1 and higher-order cognitive processes (i.e., learning, sustained attention, and long-term episodic memory; McLaurin et al. 2019a) is of fundamental importance, as these analyses suggest that the enhancement of PPI by CRISPR/Cas9 may lead to the mitigation of HIV-1-associated neurocognitive impairments more broadly.
Although the present proof-of-concept studies were planned systematically, a few caveats must be recognized. First, CRISPR/Cas9 treatment conditions, including both dose and time, need to be further optimized both in vitro and in vivo. Of particular import are time-dependent in vitro experiments and longitudinal in vivo studies to investigate whether there is an opportunity for reactivation after a period. Second, evaluation of HIV-1 viral genome eradication in vitro and in vivo was limited to the PFC. Despite the strong rationale for examining the PFC (i.e., high HIV-1 viral protein expression (Li et al. 2021); involvement in higher-order cognitive processes (Fuster 2008)), the brain is a highly interconnected organ necessitating additional studies focused on a diverse array of regions.
Taken together, proof-of-concept studies demonstrate the susceptibility of mixed glia to efficacious gene editing via CRISPR/Cas9 in vitro and in vivo. Fundamental challenges for the complete eradication of the HIV-1 viral genome remain, however, as incomplete excision and significant individual variability were observed. Nevertheless, the clinical relevance of CRISPR/Cas9 cannot be understated, as the knockdown of HIV-1 mRNA protractedly restores the developmental trajectory of temporal processing. Thus, even in the absence of full viral eradication, gene editing via CRISPR/Cas9 may afford a novel therapeutic strategy for HAND.
References
Abbondanzo SJ, Chang SL (2014) HIV-1 transgenic rats display alterations in immunophenotype and cellular responses associated with aging. PLoS ONE 9:e105256. https://doi.org/10.1371/journal.pone.0105256
Ait-Ammar A, Kula A, Darcis G, Verdikt R, De Wit S, Gautier V, Mallon PWG, Marcello A, Rohr O, Van Lint C (2020) Current status of latency reversing agents facing the heterogeneity of HIV_1 cellular and tissue reservoirs. Front Microbiol 10:3060. https://doi.org/10.3389/fmicb.2019.03060
Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, Marconi V (2019) HIV infection and incidence of cardiovascular diseases: an analysis of a large healthcare database. J Am Heart Assoc 8:e012241. https://doi.org/10.1161/JAHA.119.012241
Bhowmik R, Chaubey B (2022) CRISPR/Cas9: a tool to eradicate HIV-1. AIDS Res Ther 19:58. https://doi.org/10.1186/s12981-022-00483-y
Bohlen CJ, Bennett FC, Tucker AF, Collins HY, Mulinyawe SB, Barres BA (2017) Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron 94:759–773e8. https://doi.org/10.1016/j.neuron.2017.04.043
Caldeira C, Oliveira AF, Cunha C, Vaz AR, Falcão, Fernandes A, Brites D (2014) Microglia change from a reactive to an age-like phenotype with the time in culture. Front Cell Neurosci 8:152. https://doi.org/10.3389/fncel.2014.00152
Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS (1997) Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA 94:13193–13197. https://doi.org/10.1073/pnas.94.24.13193
Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R (2016) HIV reservoirs: what, where and how to target them. Nat Rev Microbiol 14:55–60. https://doi.org/10.1038/nrmicro.2015.5
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339:819–823. https://doi.org/10.1126/science.1231143
Cristóvão AC, Saavedra A, Fonseca CP, Campos F, Duarte EP, Baltazar G (2010) Microglia of rat ventral midbrain recovers is resting state over time in vitro: let microglia rest before work. J Neurosci Res 88:552–562. https://doi.org/10.1002/jnr.22219
Cysique LA, Maruff P, Brew BJ (2004) Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol 10:350–357. https://doi.org/10.1080/13550280490521078
Dash PK, Kaminski R, Bella R, Su H, Mathews S, Ahooyi TM, Chen C, Mancuso P, Sariyer R, Ferrante P, Donadoni M, Robinson JA, Sillman B, Lin Z, Hilaire JR, Banoub M, Elango M, Gautam N, Mosley RL, Poluektova LY, McMillan J, Bade AN, Gorantla S, Sariyer IK, Burdo TH, Young WB, Amini S, Gordon J, Jacobson JM, Edagwa B, Khalili K, Gendelman HE (2019) Sequential LASER ART and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat Commun 10:2753. https://doi.org/10.1038/s41467-019-10366-y
Denaro F, Benedetti F, Worthington MD, Scapagnini G, Krauss CC, Williams S, Bryant J, Davis H, Latinovic OS, Zella D (2020) The HIV-1 transgenic rat: relevance for HIV noninfectious comorbidity research. Microorganisms 8:1643. https://doi.org/10.3390/microorganisms8111643
Denenberg VH (1984) Some statistical and experimental considerations in the use of the analysis-of-variance procedure. Am J Physiol 246:R403–R408. https://doi.org/10.1152/ajpregu.1984.246.4.R403
Ebina H, Misawa N, Kanemura Y, Koyanagi Y (2013) Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep 3:2510. https://doi.org/10.1038/srep02510
Eisele E, Siliciano RF (2012) Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 37:377–388. https://doi.org/10.1016/j.immuni.2012.08.010
Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF (1997) Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. https://doi.org/10.1126/science.278.5341.1295
Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK (2009) Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol 27:59–65. https://doi.org/10.1038/nbt.1515
Fuster JM (2008) The prefrontal cortex, 4 edn. Academic Press, London
Gama L, Abreu CM, Shirk EN, Price SL, Li M, Laird GM, Metcalf Pate KA, Wietgrefe SW, O’Connor SL, Pianowski L, Haase AT, Van Lint C, Siliciano RF, Clements JE, LRA-SIV Study Group (2017) Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS 31:5–14. https://doi.org/10.1097/QAD.0000000000001267
Gantner P, Buranapraditkun S, Pagliuzza A, Dufour C, Pardons M, Mitchell JL, Kroon E, Sacdalan C, Tulmethakaan N, Pinyakorn S, Robb ML, Phanuphak N, Ananworanich J, Hsu D, Vasan S, Trautmann L, Fromentin R, Chomont N (2023) HIV rapidly targets a diverse pool of CD4+ T cells to establish productive and latent infections. Immunity 56:653–668e5. https://doi.org/10.1016/j.immuni.2023.01.030
Garvey LJ, Yerrakalva D, Winston A (2009) Correlations between computerized battery testing and a memory questionnaire for identification of neurocognitive impairment in HIV type 1-infected subjects on stable antiretroviral therapy. AIDS Res Hum Retrovir 25(8):765–769. https://doi.org/10.1089/aid.2008.0292
Gasiunas G, Barrangou R, Horvath P, Siksnys V (2012) Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc Natl Acad Sci USA 109:E2579–2586. https://doi.org/10.1073/pnas.1208507109
Geng G, Liu B, Chen C, Wu K, Liu J, Zhang Y, Pan T, Li J, Yin Y, Zhang J, Huang F, Yu F, Chen J, Ma X, Zhou J, Kuang E, Liu C, Cai W, Zhang H (2016) Development of an attenuated Tat protein as a highly-effective agent to specifically activate HIV-1 latency. Mol Ther 24:1528–1537. https://doi.org/10.1038/mt.2016.117
Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E, Jaeger BN, O’Connor C, Fitzpatrick C, Pasillas MP, Pena M, Adair A, Gonda DD, Levy ML, Ransohoff RM, Gage FH, Glass CK (2017) An environment-dependent transcriptional network specifies human microglia identity. Science 356:eaal3222. https://doi.org/10.1126/science.aal3222
Gott C, Gates T, Dermody N, Brew BJ, Cysique LA (2017) Cognitive change trajectories in virally suppressed HIV-infected individuals indicate high prevalence of disease activity. PLoS ONE 12:e0171887. https://doi.org/10.1371/journal.pone.0171887
Gray SJ, Matagne V, Bachaboina L, Yadav S, Ojeda SR, Samulski RJ (2011) Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther 19:1058–1069. https://doi.org/10.1038/mt.2011.72
Gruell H, Gunst JD, Cohen YZ, Pahus MH, Malin JJ, Platten M, Millard KG, Tolstrup M, Jones RB, Conce Alberto WD, Lorenzi JCC, Oliveira TY, Kümmerle T, Suárez I, Unson-O’Brien C, Nogueira L, Olesen R, Østergaard L, Nielsen H, Lehmann C, Nussenzweig MC, Fätkenheuer G, Klein F, Caskey M, Søgaard OS (2022) Effect of 3BNC117 and romidepsin on the HIV-1 reservoir in people taking suppressive antiretroviral therapy (ROADMAP): a randomised, open-label, phase 2A trial. Lancet Microbe 3:e203–e214. https://doi.org/10.1016/S2666-5247(21)00239-1
Gruntman AM, Su L, Flotte TR (2017) Retro-orbital venous sinus delivery of rAAV9 mediates high-level transduction of brain and retina compared with temporal vein delivery in neonatal mouse pups. Hum Gene Ther 28:228–230. https://doi.org/10.1089/hum.2017.037
Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, Richman DD, Valentine FT, Jonas L, Meibohm A, Emini EA, Chodakewitz JA (1997) Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med 337:734–739. https://doi.org/10.1056/NEJM199709113371102
Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M, Martinez-Picado J, Nijhuis M, Wensing AMJ, Lee H, Grant P, Nastouli E, Lambert J, Pace M, Salasc F, Monit C, Innes AJ, Muir L, Waters L, Frater J, Lever AM, Edwards SG, Gabriel IH, Olavarria E (2019) HIV-1 remission following CCR5∆32/∆32 haematopoietic stem-cell transplantation. Nature 568:244–248. https://doi.org/10.1038/s41586-019-1027-4
Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, CHARTER Group (2011) HNRC Group HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16. https://doi.org/10.1007/s13365-010-0006-1
Heaton RK, Franklin DR, Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP, Vaida F, Atkinson JH, Marcotte TD, McCutchan JA, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, Abramson I, Gamst AC, Fennema-Notestine C, Smith DM, Grant I, CHARTER Group (2015) Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dise 60:473–480. https://doi.org/10.1093/cid/ciu862
Hoffman HS, Searle JL (1965) Acoustic variables in the modification of startle reaction in the rat. J Comp Physiol Psychol 60:53–58. https://doi.org/10.1037/h0022325
Hsu J, Van Besien K, Glesby MJ, Phawa S, Coletti A, Warshaw MG, Petz L, Moore TB, Chen YH, Pallikkuth S, Dhummakupt A, Cortado R, Golner A, Bone F, Baldo M, Riches M, Mellors JW, Tobin NH, Browning R, Persaud D, Bryson Y International Maternal Pediatric Adolescent AIDS Clinical Traisl Network (IMPAACT) P1107 team (2023) HIV-1 remission and possible cure in a woman after haplo-cord blood transplant. Cell 186: 1115–1126e8. https://doi.org/10.1016/j.cell.2023.02.030
Hu W, Kaminski R, Yang F, Zhang Y, Cosentino L, Li F, Luo B, Alvarez-Carbonell D, Garcia-Mesa Y, Karn J, Mo X, Khalili K (2014) RNA-directed gene editing specifically eradicates latent and prevents new HIV-1 infection. Proc Natl Acad Sci USA 111:11461–11466. https://doi.org/10.1073/pnas.1405186111
Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K, Schneider T, Hofman J, Kücherer C, Blau O, Blau IW, Hofmann WK, Thiel E (2009) Long-term control of HIV by CCR5 Delta 32/Delta32 stem-cell transplantation. N Engl J Med 360:692–698. https://doi.org/10.1056/NEJMoa0802905
Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A (1987) Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol 169:5429–5433. https://doi.org/10.1128/jb.169.12.5429-5433.1987
Ison JR, Hammond GR (1971) Modification of the startle reflex in the rat by changes in the auditory and visual environments. J Comp Physiol Psychol 75:435–452. https://doi.org/10.1037/h0030934
Jensen BEO, Knops E, Cords L, Lübke N, Salgado M, Busman-Sahay K, Estes JD, Huyveneers LEP, Perdomo-Celis F, Wittner M, Gálvez C, Mummert C, Passaes C, Eberhard JM, Münk C, Hauber I, Hauber J, Heger E, De Clercq J, Vandekerckhove L, Bergmann S, Dunay GA, Klein F, Häussinger D, Fischer JC, Nachtkamp K, Timm J, Kaiser R, Harrer T, Luedde T, Nijhuis M, Sáez-Cirión A, Schulze Zur Wiesch J, Wensing AM, Martinez-Picado J, Kobbe G (2023) In-depth virological and immunological characterization of HIV-1 cure after CCR5∆32/∆32 allogeneic hematopoietic stem cell transplantation. Nat Med 29:583–587. https://doi.org/10.1038/s41591-023-02213-x
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012) A programmable dual-RNA_guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821. https://doi.org/10.1126/science.1225829
Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J (2013) RNA-programmed genome editing in human cells. Elife 2:e00471. https://doi.org/10.7554/eLife.00471
Kaminski R, Chen Y, Fischer T, Tedaldi E, Napoli A, Zhang Y, Karn J, Hu W, Khalili K (2016a) Elimination of HIV-1 genomes from human T-lymphoid cells by CRISPR/Cas9 gene editing. Sci Rep 6:22555. https://doi.org/10.1038/srep22555
Kaminski R, Bella R, Yin C, Otte J, Ferrante P, Gendelman HE, Li H, Booze R, Gordon J, Hu W, Khalili K (2016b) Excision of HIV-1 DNA by gene editing: a proof-of-concept in vivo study. Gene Ther 23:690–695. https://doi.org/10.1038/gt.2016.41
Ko A, Kang G, Hattler JB, Galadima HI, Zhang J, Li Q, Kim WK (2019) Macrophages but not astrocytes harbor HIV DNA in the brains of HIV-1-infected aviremic individuals on suppressive antiretroviral therapy. J Neuroimmune Pharmacol 14:110–119. https://doi.org/10.1007/s11481-018-9809-2
Li H, Illenberger JM, McLaurin KA, Mactutus CF, Booze RM (2018) Identification of dopamine D1-alpha receptor within rodent nucleus accumbens by an innovative RNA in situ detection technology. J Vis Exp 13357444. https://doi.org/10.3791/57444
Li H, McLaurin KA, Illenberger JM, Mactutus CF, Booze RM (2021) Microglial HIV-1 expression: role in HIV-1 associated neurocognitive disorders. Viruses 13:924. https://doi.org/10.3390/v13050924
Li H, McLaurin KA, Mactutus CF, Booze RM (2023) Microglia proliferation underlies synaptic dysfunction in the prefrontal cortex: implications for the pathogenesis of HIV-1-associated neurocognitive and affective alterations. J Neurovirol [Online Ahead of Print]. https://doi.org/10.1007/s13365-023-01147-x
Maina EK, Mureithi H, Adan AA, Muriuki J, Lwembe RM, Bukusi EA (2020) Incidences and factors associated with viral suppression or rebound among HIV patients on combination antiretroviral therapy from three counties in Kenya. Int J Infect Dis 97:151–158. https://doi.org/10.1016/j.ijid.2020.05.097
Makarova KS, Aravind L, Grishin NV, Rogozin IB, Koonin EV (2002) A DNA repair system specific for thermophilic Archaea and bacteria predicted by genomic context analysis. Nucleic Acids Res 30:482–496. https://doi.org/10.1093/nar/30.2.482
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM (2013) RNA-guided Hum Genome Eng via Cas9 Sci 339:823–826. https://doi.org/10.1126/science.1232033
Marcus JL, Leyden WA, Alexeef SE, Anderson AN, Hechter RC, Hu H, Lam JO, Towner WJ, Yuan Q, Horberg MA, Silverberg MJ (2020) Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000–2016. JAMA Netw Open 3:e207954. https://doi.org/10.1001/jamanetworkopen.2020.7954
McLaurin KA, Booze RM, Mactutus CF (2016) Progression of temporal processing deficits in the HIV-1 transgenic rat. Sci Rep 6:32831. https://doi.org/10.1038/srep32831
McLaurin KA, Booze RM, Mactutus CF (2017a) Temporal processing demands in the HIV-1 transgenic rat: amodal gating and implications for diagnostics. Int J Dev Neurosci 57:12–20. https://doi.org/10.1016/j.ijdevneu.2016.11.004
McLaurin KA, Booze RM, Mactutus CF (2017b) Selective developmental alterations in the HIV-1 transgenic rat: opportunities for diagnosis of pediatric HIV-1. J Neurovirol 23:87–98. https://doi.org/10.1007/s13365-016-0476-x
McLaurin KA, Cook AK, Li H, League AF, Mactutus CF, Booze RM (2018a) Synaptic connectivity in medium spiny neurons of the nucleus accumbens: a sex-dependent mechanism underlying apathy in the HIV-1 transgenic rat. Front Behav Neurosci 12:285. https://doi.org/10.1093/cid/ciab484
McLaurin KA, Booze RM, Mactutus CF (2018b) Evolution of the HIV-1 transgenic rat: utility in assessing the progression of HIV-1-associated neurocognitive disorders. J Neurovirol 24:229–245. https://doi.org/10.1007/s13365-017-0544-x
McLaurin KA, Mactutus CF, Booze RM, Fairchild AJ (2019a) An empirical mediation analysis of mechanisms underlying HIV-1-associated neurocognitive disorders. Brain Res 1724:146436. https://doi.org/10.1016/j.brainres.2019.146436
McLaurin KA, Moran LM, Li H, Booze RM, Mactutus CF (2019b) The power of interstimulus interval for the assessment of temporal processing in rodents. J Vis Exp 14610.3791/58659
McLaurin KA, Li H, Booze RM, Mactutus CF (2019c) Disruption of timing: NeuroHIV progression in the post-cART era. Sci Rep 9:827. https://doi.org/10.1038/s41598-018-36822-1
McLaurin KA, Li H, Booze RM, Mactutus CF (2021) Neurodevelopmental processes in the prefrontal cortex derailed by chronic HIV-1 viral protein exposure. Cells 10:3037. https://doi.org/10.3390/cells10113037
Minassian A, Henry BL, Woods SP, Vaida F, Grant I, Geyer MA, Perry W, Translational Methamphetamine AIDS Research Center (TMARC) Group (2013) Prepulse inhibition in HIV-associated neurocognitive disorders. J Int Neuropsychol Soc 19:709–717. https://doi.org/10.1017/S1355617713000301
Montilla A, Zabala A, Matute C, Domercq M (2020) Functional and metabolic characterization of microglia culture in a defined medium. Front Cell Neurosci 14:22. https://doi.org/10.3389/fncel.2020.00022
Moore DJ, Masliah E, Rippeth JD, Gonzalez R, Carey CL, Cherner M, Ellis RJ, Achim CL, Marcotte TD, Heaton RK, Grant I, HNRC Group (2006) Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 20:879–887. https://doi.org/10.1097/01.aids.0000218552.69834.00
Morales DR, Moreno-Martos D, Matin N, McGettigan P (2022) Health conditions in adults with HIV compared with the general population: a population-based cross-sectional analysis. EClinicalMedicine 47:101392. https://doi.org/10.1016/j.eclinm.2022.101392
Moran LM, Booze RM, Mactutus CF (2013) Time and time again: temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J Neuroimmune Pharmacol 8:988–997. https://doi.org/10.1007/s11481-013-9472-6
Ngnitewe Massa R, Minutello K, Mesfin FB (2022) Neuroanatomy, cavernous sinus. StatPearls [Internet]. StatPearls Publishing: Treasure Island, FL
Paxinos G, Watson C (2014) The rat brain in stereotaxic coordinates, 7th edn. Elsevier Academic Press, Cambridge, MA
Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL (2010) The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol 218:94–101. https://doi.org/10.1016/j.jneuroim.2009.09.014
Prabhakar S, Cheah PS, Zhang X, Zinter M, Gianatasio M, Hudry E, Bronson RT, Kwiatkowski DJ, Stemmer-Rachamimov A, Magquire CA, Sena-Esteves M, Tannous BA, Breakefield XO (2019) Long-term therapeutic efficacy of intravenous AAV-mediated hamartin replacement in mouse model of tuberous sclerosis type 1. Mol Ther Methods Clin Dev 15:18–26. https://doi.org/10.1016/j.omtm.2019.08.003
Prabhakar S, Lule S, da Hora CC, Breakefield XO, Cheah PS (2021) AAV9 transduction mediated by systemic delivery of vector via retro-orbital injection in newborn, neonatal and juvenile mice. Exp Anim 70:450–458. https://doi.org/10.1538/expanim.20-0186
Puck TT, Cieciura SJ, Robinson A (1958) Genetics of somatic mammalian cells. III. Long term cultivation of euploid cells from human and animal subjects. J Exp Med 108:945–956. https://doi.org/10.1084/jem.108.6.945
Qu X, Wang P, Ding D, Li L, Wang H, Ma L, Zhou X, Liu S, Lin S, Wang X, Zhang G, Liu S, Liu L, Wang J, Zhang F, Lu D, Zhu H (2013) Zinc-finger-nucleases mediate specific and efficient excision of HIV-1 proviral DNA from infected and latently infected human T cells. Nucleic Acids Res 41:7771–7782. https://doi.org/10.1093/nar/gkt571
Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Østergaard L, Søgaard OS Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. E13–E21. https://doi.org/10.1016/S2352-3018(14)70014-1
Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Hayes N, Jone O, Doodnauth D, Davis H, Sill A, O’Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J (2001) An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci USA 98:9271–9276. https://doi.org/10.1073/pnas.161290298
Réu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Druid H, Frisén J (2017) The lifespan and turnover of microglia in the human brain. Cell Rep 20:779–784. https://doi.org/10.1016/j.celrep.2017.07.004
Roscoe RF, Mactutus CF, Booze RM (2014) HIV-1 transgenic female rat: synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J Neuroimmune Pharmacol 9:642–653. https://doi.org/10.1093/cid/ciab484
Rubin LH, Maki PM, Springer G, Benning L, Anastos K, Gustafson D, Villacres MC, Jiang X, Adimora AA, Waldrop-Valverde D, Vance DE, Bolivar H, Alden C, Martin EM, Valcour VG, Women’s Interagency HIV Study (2017) Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology 89:1594–1603. https://doi.org/10.1212/WNL.0000000000004491
Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, Ragin A, Levine A, Miller E (2016) Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 86:334–340. https://doi.org/10.1212/WNL.0000000000002277
Souza DG, Bellaver B, Souza DO, Quincozes-Santos A (2013) Characterization of adult rat astrocyte cultures. PLoS ONE 8(3):e60282. https://doi.org/10.1371/journal.pone.0060282
Steel CD, Stephens AL, Hahto SM, Singletary SJ, Ciavarra RP (2008) Comparison of the lateral tail vein and the retro-orbital venous sinus as routes of intravenous drug delivery in a transgenic mouse models. Lab Anim (NY) 37:26–32. https://doi.org/10.1038/laban0108-26
Thompson KA, Cherry CL, Bell JE, McLean CA (2011) Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol 179:1623–1629. https://doi.org/10.1016/j.ajpath.2011.06.039
Touloumi G, Kalpourtzi N, Papastamopoulos V, Paparizos V, Adamis G, Antoniadou A, Chini M, Karakosta A, Makrilakis K, Gavana M, Vantarakis A, Psichogiou M, Metallidis S, Sipsas NV, Sambatakou H, Hadjichristodoulou C, Voulgari PV, Chrysos G, Gogos C, Chlouverakis G, Tripsianis G, Alamanos Y, Stergiou G, AMACS, EMENO (2020) Cardiovascular risk factors in HIV infected individuals: comparison with general adult control population in Greece. PLoS ONE 15:e0230730. https://doi.org/10.1371/journal.pone.0230730
Vigorito M, Connaghan KP, Chang SL (2015) The HIV-1 transgenic rat model of neuroHIV. Brain Behav Immun 48:336–349. https://doi.org/10.1016/j.bbi.2015.02.020
Wallet C, De Rovere M, Van Assche J, Daouad F, De Wit S, Gautier V, Mallon PWG, Marcello A, Van Lint C, Rohr O, Schwartz C (2019) Microglial cells: the main HIV-1 reservoir in the brain. Front Cell Infect Microbiol 9:362. https://doi.org/10.3389/fcimb.2019.00362
Wang G, Zhao N, Berkhout B, Das AT (2016a) CRISPR-Cas9 can inhibit HIV-1 replication but NHEJ repair facilitates virus escape. Mol Ther 24:522–526. https://doi.org/10.1038/mt.2016.24
Wang Z, Pan Q, Gendron P, Zhu W, Guo F, Cen S, Wainberg MA, Liang C (2016b) CRISPR/Cas9-derived mutations both inhibit HIV-1 replication and accelerate viral escape. Cell Rep 15:481–489. https://doi.org/10.1016/j.celrep.2016.03.042
Wang Y, Liu M, Lu Q, Farrell M, Lappin JM, Shi J, Lu L, Bao Y (2020) Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology 95:e2610–e2621. https://doi.org/10.1212/WNL.0000000000010752
Wears RL (2002) Advanced statistics: statistical methods for analyzing cluster and cluster-randomized data. Acad Emerg Med 9:330–341. https://doi.org/10.1111/j.1553-2712.2002.tb01332.x
Weiss JJ, Calvi R, Naganawa M, Toyonaga T, Farhadian SF, Chintanaphol M, Chiarella J, Zheng MQ, Ropchan J, Huang Y, Pietrzak RH, Carson RE, Spudich S (2021) Preliminary in vivo evidence of reduced synaptic density in human immunodeficiency virus (HIV) despite antiretroviral therapy. Clin Infect Dis 73:1404–1411. https://doi.org/10.1093/cid/ciab484
Williams DW, Eugenin EA, Calderon TM, Berman JW (2012) Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J Leukoc Biol 91:401–415. https://doi.org/10.1189/jlb.0811394
Wong JK, Hezareh M, Günthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD (1997) Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291–1295. https://doi.org/10.1126/science.278.5341.1291
Xue C, Greene EC (2021) DNA repair pathway choices in CRISPR-Cas9-mediated genome editing. Trends Genet 37:639–656. https://doi.org/10.1016/j.tig.2021.02.008
Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S (2011) Retro-orbital injections in mice. Lab Anim (NY) 40:155–160. https://doi.org/10.1038/laban0511-155
Yedavalli VSRK, Benkirane M, Jeang KT (2003) Tat and trans-activation-responsive (TAR) RNA-independent induction of HIV-1 long terminal repeat by human and murine cyclin T1 requires Sp1. J Biol Chem 278:6404–6410. https://doi.org/10.1074/jbc.M209162200
Yin C, Zhang T, Li F, Yang F, Putatunda R, Young WB, Khalili K, Hu W, Zhang Y (2016) Functional screening of guide RNAs targeting the regulatory and structural HIV-1 viral genome for a cure of AIDS. AIDS 30:1163–1174. https://doi.org/10.1097/QAD.0000000000001079
Yin C, Zhang T, Qu X, Zhang Y, Putatunda R, Xiao X, Li F, Xiao W, Zhao H, Dai S, Qin X, Mo X, Young WB, Khalili K, Hu W (2017) In vivo excision of HIV-1 provirus by saCas9 and multiplex single-guide RNAs in animal models. Mol Ther 25:1168–1186. https://doi.org/10.1016/j.ymthe.2017.03.012
Acknowledgements
This work was supported in part by grants from NIH (National Institute on Drug Abuse, R01-DA013137; National Institute on Drug Abuse, K99-DA056288; National Institute on Drug Abuse, R01-DA059310; National Institute of Mental Health, R01-MH106392; National Institute of Neurological Disorders and Stroke, R01-NS100624).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K.K. is named as an inventor on patents that cover the viral gene editing technology that is the subject of this article. In addition, K.K. is a co-founder, board member (observer), and chief scientific adviser and holds equity in Excision Biotherapeutics, a biotech startup that has licensed the viral gene editing technology from Temple University for commercial development and clinical trials. All other authors have no interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McLaurin, K.A., Li, H., Khalili, K. et al. HIV-1 mRNA knockdown with CRISPR/CAS9 enhances neurocognitive function. J. Neurovirol. 30, 71–85 (2024). https://doi.org/10.1007/s13365-024-01193-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-024-01193-z