Abstract
The aim of the study was to evaluate the incidence of brain opportunistic pathologies and survival in patients living with HIV from a Romanian tertiary center. A 15-year prospective observational study of brain opportunistic infections diagnosed in HIV-infected patients was performed at Victor Babes Hospital, Bucharest, between January 2006 and December 2021. Characteristics and survival were compared related to modes of HIV acquisition and type of opportunistic infection. A total of 320 patients were diagnosed with 342 brain opportunistic infections (incidence 9.79 per 1000 person-years), 60.2% males with median age at diagnosis of 31 years (IQR 25, 40). Median CD4 cell count and VL were 36/μL (IQR 14, 96) and 5.1 log10 copies/mL (IQR 4, 5.7) respectively. The routes of HIV acquisition were heterosexual (52.6%), parenteral route in early childhood (31.6%), injecting drug use (12.9%), men having sex with men (1.8%), and vertical (1.2%). The most common brain infections were progressive multifocal leukoencephalopathy (31.3%), cerebral toxoplasmosis (26.9%), tuberculous meningitis (19.3%), and cryptococcal meningitis (16.7%). Patients infected by parenteral mode in early childhood were younger at diagnosis of both opportunistic infection and HIV (p < 0.001 and p < 0.001, respectively), developed more frequently PML (p < 0.001), and had the lowest early (p = 0.002) and late (p = 0.019) mortality rates. Risk factors for shorter survival were age > 30 years (p = 0.001), injecting drug use (p = 0.003), CD4 + < 100/μL (p = 0.007), and VL > 5 log10 copies/mL at diagnosis (p < 0.001). The incidence and mortality rate of brain opportunistic infections were high and did not decrease significantly during the study period, due to late presentation or non-adherence to ART.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Despite the wide availability of antiretroviral treatment (ART), AIDS-related conditions still represent an important challenge for clinicians. The high number of people living with HIV/AIDS (PLWH) diagnosed with severe immunosuppression (late presenters), poor linkage to care, and lack of adherence to ART which leads to HIV drug resistance, as well as lifestyle factors, result in poor outcomes and high mortality rates in some PLWH (Coelho et al. 2016; von Wyl et al. 2013).
Numerous studies suggest that the brain is one of the most affected organs in PLWH (Ondounda et al. 2016; Gray et al. 2003). Persistence of viral reservoirs in nervous tissue and poor penetration of HIV drugs into the brain are challenging factors in managing patients with neurological complications. Due to a strong neuroimmune response induced by the virus, PLWH often experience chronic inflammation followed by cognitive decline and complications like HIV-associated neurocognitive disorders (HAND) or central nervous system opportunistic infections (CNS-OIs). HIV neurotropism and neurovirulence are also considered risk factors for the high prevalence of CNS-OIs and tumors with CNS involvement (Dunfee et al. 2006).
The most frequent diagnosed CNS-OIs are tuberculous meningitis (TBM), cerebral toxoplasmosis (CNS toxo), progressive multifocal leukoencephalopathy (PML), cryptococcal meningitis, and CMV encephalitis (CMVE). In addition, primary cerebral lymphoma (PCNSL) is part of the differential diagnosis, especially in immunosuppressed patients (Bowen et al. 2016; Manzardo et al. 2005; Nael et al. 2016; Sheybani et al. 2021; Mhatre et al. 2020).
Due to efficient ART, the overall incidence of HIV-related neuroinfections has significantly declined during the last decade. However, CNS-OIs remain an important cause of morbidity and mortality, especially in resource-limited settings (Bowen et al. 2016; Sheybani et al. 2021; Hanif et al. 2022). The diagnosis can sometimes be difficult due to inconclusive brain imaging and cerebrospinal fluid (CSF) findings, and because brain biopsy cannot be always performed (Albarillo and O'Keefe 2016; Wright 2014; Mulherkar et al. 2022). Furthermore, immunosuppressed patients who initiate ART may develop immune reconstitution inflammatory syndrome (IRIS) associated with high morbidity and sometimes with death (Manzardo et al. 2005).
An increasing number of reports in the literature suggest reduced incidence of CNS-OIs in the ART era comparing to pre-ART period (Gray et al. 2003; Matinella et al. 2015; UKCHCSS Committee 2011). However, severe immunosuppression, older age, illegal drug use, and hepatitis C co-infection are all factors associated with a higher risk of neurocognitive impairment and CNS opportunistic infections in treated individuals (Mulherkar et al. 2022; McNamara et al. 2017; Valcour et al. 2006; Nath 2010; Parsons et al. 2006).
Romania is a Central-East European country with low HIV prevalence, estimated to be below 0.1% (CNLAS 2022). According to the National Report from December 2022, a total of 27,077 patients were diagnosed with HIV since 1985, of whom 17,923 are still alive (CNLAS 2021). Almost half of them acquired HIV infection by parenteral mode, in the 1980s during their first years of life, due to unsterilized injection equipment or transfusions. This is a large homogenous cohort of patients infected with the F1 clade (phylogenetically linked to the Angolan F subtype) that was considered at one point to be more neurotropic (Ene et al. 2014; CNLAS 2015; Apetrei et al. 1998; Guimaraes et al. 2009). Currently, report highlighted that heterosexual transmission is the most common mode of HIV acquisition in Romania (60%) and that there is an increase of newly HIV diagnosed persons among men having sex with men (MSM) (30% in the newly diagnosed cases). Injecting drug users are still at high risk of HIV transmission in Romania, but the prevalence of HIV among these patients has shown a decrease since 2014, when the new psychoactive drugs (NPS), known as “ethnobotanicals,” considered to be responsible for the HIV epidemic among people who inject drugs (PWIDs), were declared illegal (CNLAS 2016; EMCDDA 2016; Mărdărescu et al. 2021).
Studies regarding CNS-OIs in HIV-infected patients in Romania are scarce. One report from different regions in the country revealed a high frequency of PML and cerebral toxoplasmosis (3rd CE HIV Forum 2016).
Our study is among the first performed in Romania, focused on PLWH who developed CNS-OIs over 15 years. It describes a large cohort of patients, identifying risk factors for poor evolution and high mortality rates. It shows a real image of how the HIV epidemic in this region was influenced by the use of efficient ART and it signals risk factors for CNS-OIs development, as well as issues that need to be improved in order to reduce the morbidity and mortality in PLWH.
Objective
The aim of the study was to assess the incidence over time, socio-demographic, clinical characteristics, and survival with CNS-OIs (including brain tumors) in PLWH from a tertiary health care facility in Bucharest, Romania.
Methods
Patient population
We performed a prospective study on PLWH diagnosed with CNS-OIs at Victor Babes Clinical Hospital for Infectious and Tropical Diseases in Bucharest between 1st January 2006 and 31st December 2021. All patients were followed from the date of CNS-OI diagnosis (“baseline”) until their death, or for those who survived until the end of December 2021.
We collected demographic and clinical data, including gender, age at diagnosis of both HIV and CNS-OI, immuno-virologic variables (nadir CD4, CD4 cell count, and HIV viral load), treatment regimens, and survival in patients with CNS toxo, cryptococcal meningitis, TBM, PML, CMVE, and PCNSL.
The study was approved by the hospital ethics committee and all patients signed informed consent at their admission.
Laboratory methods
Diagnosis of CNS-OIs was considered definitive on positive microbiological examination of the CSF or brain biopsy, and presumptive in patients with typical clinical features and magnetic resonance imaging (MRI) brain scanning imaging.
The Laboratory diagnosis was based on CSF bacterial, fungal, or mycobacterial cultures, India ink stains (for Cryptococcus neoformans) or acid-fast bacilli stains (for TBM), detection of Cryptococcal antigen, cells count, proteins and glucose concentrations, cytology, and flow cytometry as well as PCR testing for JC virus, EBV, CMV, Toxoplasma gondii, and Mycobacterium tuberculosis.
In the 2 last years of the study, two types of multiplex PCR for microbial detection in CSF (from BioFire and Qiagen) were used for the diagnosis of bacterial infections (E. coli, Listeria monocytogenes , Haemophilus influenzae, Neisseria meningitidis, Streptococcus agalactiae, Streptococcus pneumoniae), viruses (CMV, HSV, HHV6, VZV), and yeast (Cryptococcus neoformans and Cryptococcus gatii).
CD4 cell counts were determined by flow cytometry using the four-color reagent CD3-FITC/CD8-PE/CD45-PerCP/CD4-APC (Becton Dickinson, San Jose, CA, USA) on a FACS Calibur and FACS Canto II Becton Dickinson flow cytometers. HIV viral load was determined using the COBAS AmpliPrep/COBAS TaqMan HIV-1 Test Version 2.0, Roche, which has a lower detection limit of 16.5 copies/mL.
According to the international definitions, patients with a CD4 cell count below 350/μL at the time of HIV diagnosis were considered late presenters (LP), while patients with a CD4 cell count < 200/μL with advanced HIV disease (AHD) (Antinori et al. 2011).
Statistical analysis
The risk of a CNS-OIs in each calendar year of the study period was calculated by dividing the number of patients newly diagnosed with a CNS-OI by the total number of HIV-positive individuals that attended Victor Babes Hospital for HIV care during that year. Demographic and immuno-virologic characteristics at the time of CNS-OI diagnosis were summarized with median and interquartile range (IQR) or with count (n) and percent (%). Fisher’s exact test was used to compare proportions and rates between groups. Numeric variables were compared between groups using the Kruskal–Wallis test. p values from pairwise comparisons were adjusted using false discovery rate (FDR) method.
Analyses of survival from CNS-OI diagnosis were restricted to the first event per person. Since all patients were followed for at least 3 months post-CNS-OI diagnosis, the percentage with short-term (3-month) survival was calculated. Rates of longer-term survival were calculated by dividing the number of deaths from 3 months onwards by the total person-years of follow-up from that time point. Additionally, separate Cox proportional hazards (PH) models were used to assess survival from mode of HIV transmission, types of CNS-OIs, age, CD4 cell count, and HIV viral load at OI diagnosis. All five predictors were considered for inclusion into multivariable model. Backward model selection with significance threshold of 0.10 was used to obtain the final multivariable model. The results are presented in the form of hazard ratio (HR) and 95% confidence interval (CI95). p values and confidence intervals were FDR-adjusted where multiple comparisons took place. Visually, time from CNS-OI diagnosis to death was assessed using Kaplan–Meier plots.
Analyses were performed using the R version 4.2.1 (2022–06-23). A p value of less than 0.05 was considered to indicate a statistically significant difference.
Results
During the 15-year study period, 320 individuals were diagnosed with 342 CNS-OIs and AIDS-related brain tumors. Two or more CNS-OIs were diagnosed simultaneously in 18 patients, while 3 were relapses of the same condition. The number of PLWH under observation at our hospital increased from 931 in 2006 to 2357 in 2021. This corresponds to increased incidence risk of CNS-OI of 0.5% in 2006 to 1.06% in 2021, with a peak of 1.9% observed in 2013 (Fig. 1).
Characteristics of HIV-infected patients diagnosed with CNS-OIs
More than half of the CNS-OIs were diagnosed in males (206/342, 60.2%), with a median age of 31 years (IQR 25, 40). The median time between HIV and OI diagnoses was 1 year (IQR 0, 10), and in approximately one-third of cases, 36.0% (119/331), HIV and the CNS-OIs were diagnosed simultaneously. CNS-IRIS was diagnosed in 9 cases (8 cases with PML and 1 patient with TBM).
The routes of HIV acquisition were as follows: heterosexual transmission (52.6%), parenteral mode during childhood (31.6%), due to injecting drug use (12.9%), sex between men (1.8%), and mother-to-child transmission (1.2%) (Table 1).
Patients who developed CNS-OIs were severely immunosuppressed at OI diagnosis (median CD4 + 36 cell count/μL (IQR 14, 96) and with high HIV viral load (median HIV-RNA 5.2 log10 copies/mL (IQR 4.0, 5.7). Among patients previously diagnosed with HIV, 80.3% (143/186) were on ART at CNS-OI diagnosis and a large proportion of them (92.3% (132/143)) had a viral load of more than 50 copies/mL, likely due to non-adherence. There were 3 patients severely immunosuppressed diagnosed with TBM despite HIV viral load below 50 copies/mL (Table 1).
The most frequent CNS-OIs diagnosed during the study period were PML 31.3% (107/342), CNS toxo 26.9% (92/342), TBM 19.3% (66/342), and cryptococcal meningitis 16.7% (57/342). PCNSL was diagnosed in 3.2% (11/342) cases and 2.6% (9/342) patients developed CMV encephalitis (CMVE).
TBM with drug-resistant strains was diagnosed in 15.8% (10/63) cases, as follows: 2 patients with multidrug-resistant TB (MDR — resistance to both Isoniazid and Rifampin), 2 cases with pre-XDR TB (MDR and resistance to fluoroquinolones or second-line injectable drugs), and 6 patients with extensive drug resistance (XDR TB — MDR and resistance to both fluoroquinolones and injectables).
Figure 2 shows a significant association between the CNS-OI type and route of HIV infection (p < 0.001), with all three groups differing from each other (all pairwise ps < 0.05). Parenterally infected individuals were diagnosed more often with PML (46.3%), while PWIDs more frequently had TBM (36.4%) (p < 0.001). Cerebral toxo was common in all 3 groups, with the highest percentage (28.9%) observed in those who acquired HIV by heterosexual contact. Cryptococcal meningitis was more common in PWIDs (25.0%). The vast majority of patients diagnosed with PCNSL and CMVE acquired HIV by heterosexual contact.
Distribution of CNS-OIs by mode of HIV acquisition. 6 individuals who acquired HIV through sex between men, and 4 through vertical transmission were excluded. PCNLS, primary central nervous system lymphoma. FDR-adjusted pairwise comparisons show all 3 groups differ on the distributions of CNS-OIs (all 3 p < 0.05)
The age at diagnosis of both HIV and CNS-OIs differed by route of infection (p < 0.001), with older ages observed in patients infected via heterosexual contact, followed by those infected through injecting drug use, and the youngest ages observed in the parenterally infected patients. Among CNS-OIs, while the PCNSL was associated with older age (years) at the diagnosis of both HIV (mean age 37.2 compared to mean ages ranging 24.8 to 29.9 for other CNS-OIs) and CNS-OI (40.3 compared to 31.2–33.8), the pairwise comparisons did not reach statistical significance. The percentage of males was significantly lower (p = 0.005) for participants with CMVE (11.1%) compared to those with TBM (71.2%), cryptococcal meningitis (68.4%), and PML (60.7%).
Participants with parental and injecting drug use routes of HIV acquisition had lower CD4 cell count at diagnosis (p = 0.006) and lower nadir CD4 (p = 0.019) compared to heterosexual acquisition group. Parenterally infected participants had lower HIV-RNA at diagnosis (p = 0.004) than the HIV-RNA levels in the other two groups, as shown in Table 2.
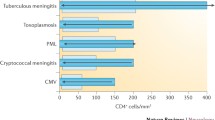
Patients with cryptococcal meningitis and CNS toxo had lower CD4 and nadir CD4 cell count at diagnosis (p < 0.001 and p = 0.004, respectively). Patients diagnosed with PML had significantly lower plasma HIV-RNA compared to all other CNS-OI groups (p < 0.001).
When considering survival from the date of the first CNS-OI diagnosis in the 320 patients, there were significant differences between participants with different routes of HIV acquisition (p = 0.003, Fig. 3A), with parenterally infected patients showing better overall survival compared to heterosexually infected patients (HR 0.47, CI95 0.26–0.88, p = 0.017) and PWIDs (HR 0.52, CI95 0.31–0.86, p = 0.005), with no significant difference between the latter two groups (HR 0.90, CI95 0.56–1.51, p = 0.674). Parenteral mode of infection was associated with the lowest overall mortality rates, significantly lower than in PWIDs (p = 0.033), and lower early deaths (14.3%) compared to heterosexual (34.3%, p = 0.002) and PWIDs (31.6%, p = 0.043) (see Table 3). Figure 3B shows Kaplan–Meier curves for CNS-OI.
The Cox PH model yielded a significant p value for comparing hazards between CNS-OI (0.001). The FDR-adjusted pairwise comparisons showed marginal significance for higher mortality associated with cryptococcal meningitis compared to CNS toxo (HR 2.04, CI95 0.99–4.20, p = 0.056) and to PML (HR 1.95, CI95 0.99–3.77, p = 0.056). Similar results were observed for TBM when compared to CNS toxo (HR 1.88, CI95 0.99–3.60, p = 0.056) and to PML (HR 1.80, CI95 0.99–3.24, p = 0.056). Combined PCNSL and CMVE group was associated with higher early mortality rate (47.1%), but without statistical significance (Table 3). In the subset of participants who survived for longer than 3 months (Table 3), higher mortality rate was associated with TBM compared to CNS toxo (p = 0.009) and to PML (p = 0.019).
Age older than 30 years (HR 1.81, CI95 1.28–2.58, p = 0.001), CD4 cell count below 100 cells/μL (HR 1.79, CI95 1.16–2.88, p = 0.007), and HIV viral load higher than 5 log10 copies/mL (HR 2.07, CI95 1.38–3.18, p < 0.001), all measured at CNS-OIs diagnosis, were factors associated with a shorter survival (Fig. 3C−E).
Backward model selection resulted in a multivariable model that excluded mode of HIV acquisition (p = 0.683). In the final model, the predictors were age older than 30 (HR 1.51, CI95 0.99–2.33, p = 0.053), CD4 cell count below 100 cells/μL (HR 1.77, CI95 1.07–3.06, p = 0.026), HIV viral load higher than 5 log10 copies/mL (HR 1.68, CI95 1.08–2.64, p = 0.020), and CNS-OIs diagnosis (overall p = 0.009). There were two significant FDR-adjusted pairwise comparisons from this model: compared to CNS toxo, higher mortality was associated with cryptococcal meningitis (HR 2.43, CI95 1.07–5.67, p = 0.027) and with TBM (HR 2.48, CI95 1.06–6.11, p = 0.027) (Fig. 3).
Discussion
In this study, we report a high incidence of CNS-OIs and AIDS-related brain tumors among PLWH from a Romanian tertiary health care facility. The incidence per year varied over time, but although the study was performed in the ART era, the number of patients diagnosed with CNS-OIs was relatively high. Late diagnosis of HIV infection and non-adherence to ART were factors associated with the development of CNS-OIs.
During the last decade, we registered a high number of patients diagnosed as late presenters or with advanced HIV disease, most often infected with HIV by heterosexual contact or due to injecting drug use, who developed opportunistic infections, AIDS-related malignancies and severe bacterial infections (Ianache et al. 2016; Oprea et al. 2017). A large number of patients in this study were diagnosed with HIV and the CNS-OI simultaneously, most of them being unaware of their HIV status. According to the European guidelines, since the end of 2015, patients from our setting initiated ART at the time of HIV diagnosis, irrespective of their CD4 cell count (EACS 2015; DHHS 2016). During the first years of our study (until 2015), ART was recommended only for all patients with CD4 count below 350/µL or those diagnosed with opportunistic infections (CNLAS 2015; ECDC 2014).
Among patients previously diagnosed with HIV, more than half were already on ART at CNS-OI diagnosis, but not adherent. A high proportion of them belonged to the Romanian cohort of patients infected with HIV by parenteral mode during their first years of life in the late 1980s. They were long-term survivors, multiple experienced to all classes of antiretroviral drugs, including the initial ones which caused more adverse events. Some of them experienced “therapeutic fatigue” responsible for poor adherence to treatment and increased risk of drug resistance (CNLAS 2015). PWIDs also tend to have very poor adherence to ART due to severe neuro-psychiatric disorders related to drug abuse. Drug-drug interactions between ART and injectable new psychoactive substances and their impact on ART effectiveness are unknown. (Ianache et al. 2016; DHHS 2016; Karila et al. 2015; Kumar et al. 2015; Nils von 2016; Sanchez et al. 2015). PWIDs also tend to avoid medical services due to fear of stigma, and are often diagnosed as late presenters with concomitant opportunistic infections (Oprea et al. 2018; Ianache et al. 2016). Because of this, PWIDs in our study had severe immunosuppression and shorter survival.
The most frequent CNS-OIs in our study were PML, diagnosed more often in parenterally infected patients, CNS toxo in heterosexuals, TBM, and cryptococcal meningitis, the latter two more frequently seen in PWIDs. We diagnosed only a few cases of PCNSL and CMVE, almost all of them in heterosexually infected patients. Data from the literature suggest that compared to other CNS-OIs, PML is associated with higher CD4 cell count, pre-exposure to ART, and previous HIV diagnosis (UKCHCSS Committee 2011; Tan et al. 2009; Venkataramana et al. 2006). The high frequency of PML in parenterally infected patients from our study, associated with higher CD4 cell count compared to other OIs, is in concordance with these data. Parenterally infected patients from our cohort were diagnosed with HIV during childhood, experienced almost all classes of antiretroviral medications and even if not consistently adherent to treatment, they had higher CD4 cell count and lower HIV viral load compared to HSX and PWIDs.
PML was also most often associated with IRIS in patients who initiated ART after CNS-OI diagnosis. Some studies highlighted that PML-IRIS occurs more often in patients with higher CD4 cell count and has greater survival rates compared with patients diagnosed with PML but no IRIS. However, PML-IRIS may be often misdiagnosed because the inflammation produced in context of HIV is lower compared to PML-IRIS that occurs in the context of other immunosuppressive conditions (Bowen et al. 2016).
We also found that injecting drug use was associated with severe immunosuppression and high HIV viral load at OI diagnosis, these results being in concordance with national reports and other studies performed in Romania which highlighted the high prevalence of late presenters and AHD among PWIDs (Ianache et al. 2016; CNLAS 2017, 2018). Romania is considered to be an endemic country for tuberculosis (TB) and vulnerable groups of patients, such as PWIDs, have a high risk of developing both pulmonary and extra pulmonary forms of TB, including infections with drug-resistant strains, explaining the high prevalence of TBM among our PWID patients (Ianache et al. 2016; Didilescu et al. 2013). Cryptococcal meningitis was associated with severe immunosuppression with low values for both nadir and median CD4 cell count at OI diagnosis, similar to other studies (CNLAS 2016; Okurut et al. 2020). Some authors have suggested that CNS toxo was more often diagnosed in patients newly diagnosed with HIV who were never exposed to antiretroviral treatment (Matinella et al. 2015).
PCNSL in PLWH still represents a common challenge for clinicians, because of poor outcome despite efficient (combined) chemotherapy and ART (Matinella et al. 2015; Bayraktar et al. 2011; Brandsma and Bromberg 2018). In our study, PCNSL was diagnosed only in a few cases, being prevalent in heterosexual patients and associated with older age at diagnosis, short survival, and high mortality rate. However, due to the low number of brain biopsies performed in HIV-positive patients in our country, we suspect that the incidence of PCNLS in these patients might be underestimated.
The overall mortality rate in our study was high, especially in patients infected by heterosexual contact. PCNSL was associated with the highest mortality rate, all deaths occurring within 3 months of diagnosis. Patients with TBM and cryptococcal meningitis had short survival and high mortality rates. The intermittent availability of serum cryptococcal antigen (CrAG) tests, first-line fungicidal (amphotericin-based) regimens for cryptococcal meningitis, rapid diagnosis tests (Xpert MTB), and drugs to treat XDR TB may have resulted in increased morbidity and mortality (Hanif et al. 2022; Okurut et al. 2020; Zeng et al. 2020).
Even though median survival was greater than 12 months for all CNS-OIs, excepting PCNSL, the early mortality rate was higher than 20%. Other studies have suggested that mortality in CNS-OIs is higher during the first year after diagnosis. The early mortality rate may be due to poor adherence to ART in the context of neurocognitive impairment determined by concomitant HIV encephalopathy (HIVE) or the high risk of CNS IRIS after ART initiation in severe immunosuppressed patients (UKCHCSS Committee 2011; Tozzi et al. 2007; Post et al. 2013a, b).
Injecting drug use, older age, low CD4 cell count, and high viral load at OI diagnosis were all associated with shorter median survival and higher mortality rates. In contrast, parenterally infected patients were younger, had higher CD4 cell count and lower viral load at OI diagnosis, and had better survival and lower mortality rates. Similar to other studies, our data showed that older age, severe immunosuppression and HIV viral load at OI diagnosis are risk factors for short survival (UKCHCSS Committee 2011).
Taking into consideration the ageing of PLWH in active care at our center and the high number of late presenters still being diagnosed, we expect that poor survival and high number of CNS-OI will persist for the foreseeable future despite effective ART being available.
Limitations
A limitation of the study is that it was performed in a single center in the capital city, Bucharest.
HIV viral load was not available for all patients at CNS-OIs diagnosis. Prevalence of CNS-OIs may have been underestimated due to the lack of brain biopsies and post-mortem histopathology exams.
The incidence of CNS-OIs in the last 2 years might be underestimated as a consequence of the COVID-19 pandemic and reduction in the numbers of hospitalized patients.
Conclusions
The incidence and mortality rate of CNS-OIs in Romanian HIV-infected patients were high and did not decrease significantly during the study period; an increased proportion was diagnosed with HIV and CNS-OI simultaneously. Severe immunosuppression, high HIV viral load, older age at OI diagnosis, and injecting drug use were all risk factors for short survival and high mortality as was late presentation and/or non-adherence to ART.
The diagnosis of cerebral OIs in HIV can be difficult due to their atypical clinical presentation and appearance on brain imaging, leading to delays in diagnosis and initiation of a specific treatment. Strengthening the diagnosis methods, better adherence, especially in vulnerable groups, and immediate ART initiation are essential in reducing the CNS-OIs.
Data availability
Derived data supporting the findings of this study (data base, statistics) are available from the corresponding author (CO) on request.
References
Albarillo F, O’Keefe P (2016) Opportunistic Neurologic Infections in Patients with Acquired Immunodeficiency Syndrome (AIDS). Curr Neurol Neurosci Rep 16(1):10
Antinori A, Coenen T, Costagiola D, Dedes N, Ellefson M, Gatell J et al (2011) Late presentation of HIV infection: a consensus definition. HIV Med 12(1):61–64
Apetrei C, Necula A, Holm-Hansen C, Loussert-Ajaka I, Pandrea I, Cozmei C et al (1998) HIV-1 diversity in Romania. AIDS 12(9):1079–1085
Bayraktar S, Bayraktar UD, Ramos JC, Stefanovic A, Lossos IS (2011) Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J Neurooncol 101(2):257–265
Bowen LN, Smith B, Reich D, Quezado M, Nath A (2016) HIV-associated opportunistic CNS infections: pathophysiology, diagnosis and treatment. Nat Rev Neurol 12(11):662–674
Brandsma D, Bromberg JEC (2018) Primary CNS lymphoma in HIV infection. Handb Clin Neurol 152:177–186
Coelho LE, Cardoso SW, Amancio RT, Moreira RI, Ribeiro SR, Coelho AB et al (2016) Predictors of opportunistic illnesses incidence in post combination antiretroviral therapy era in an urban cohort from Rio de Janeiro. Brazil BMC Infectious Diseases 16:134
CNLAS (2021) Evolutia HIV in Romania 31 decembrie 2021 [cited 2021 31 decembrie 2021]. Available from: https://www.cnlas.ro/images/doc/31122021.pdf
Committee UKCHCSS, Garvey L, Winston A, Walsh J, Post F, Porter K et al (2011) HIV-associated central nervous system diseases in the recent combination antiretroviral therapy era. Euro J Neurol 18(3):527–34
CNLAS (2016) Evolutia infectiei HIV/SIDA in Romania 31 Decembrie 2016. [cited 2017 July]. Available from: http://www.cnlas.ro/images/doc/31122016_rom.pdf
CNLAS (2017) HIV/AIDS infection in Romania 30 June 2017. [cited 2017 30 June 2017]. Available from: http://www.cnlas.ro/images/doc/30062017_eng.pdf
CNLAS (2018) Infectia HIV/SIDA in Romania - Update 2017 [cited 2018 January]. Available from: http://www.cnlas.ro/images/doc/01122017_rom.pdf
CNLAS (2015) 30 years of HIV experience in Romania. [cited 2015 30 September 2015]. Available from: http://www.cnlas.ro/images/doc/24112015_eng.pdf
CNLAS (2022) Infectia HIV/SIDA in Romania, Update 30 iunie 2022. Available from: https://www.cnlas.ro/images/doc/30062022.pdf
DHHS (2016) Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. [updated July 2016]. Available from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf
Didilescu C, Cioran N, Chiotan D, Popescu G (2013) Tuberculosis in children in Romania. Pneumologia 62(1):10–14
Dunfee R, Thomas ER, Gorry PR, Wang J, Ancuta P, Gabuzda D (2006) Mechanisms of HIV-1 neurotropism. Curr HIV Res 4(3):267–278
EACS (2015) Guidelines Version 8.0. [updated January 2017]. Available from: http://www.eacsociety.org/files/guidelines_8.0-english-revised_20160610.pdf
ECDC (2014) From Dublin to Rome: ten years of responding to HIV in Europe and Central Asia Stockholm: ECDC. Available from: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/dublin-rome-10-years-hiv-europe-central-asia.pdf
EMCDDA (2016) Drog Ana Raportul national privind situatia drogurilor 2016; Romania – noi tendinte si evolutii. [cited 2017 July]. Available from: http://www.ana.gov.ro/rapoarte%20nationale/RN_2016.pdf
Ene L, Franklin DR, Burlacu R, Luca AE, Blaglosov AG, Ellis RJ et al (2014) Neurocognitive functioning in a Romanian cohort of young adults with parenterally acquired HIV-infection during childhood. J Neurovirol 20(5):496–504
Gray F, Chretien F, Vallat-Decouvelaere AV, Scaravilli F (2003) The changing pattern of HIV neuropathology in the HAART era. J Neuropathol Exp Neurol 62(5):429–440
Guimaraes ML, Vicente AC, Otsuki K, da Silva RF, Francisco M, da Silva FG et al (2009) Close phylogenetic relationship between Angolan and Romanian HIV-1 subtype F1 isolates. Retrovirology 6:39
Hanif F, Satiti S, Subagya S, Retnowulan H, Subronto YW, Mulya DP et al (2022) Progressive Worsening of Neurological Manifestations in HIV-Associated Opportunistic Central Nervous System (CNS) Infection Patients After COVID-19 Vaccinations: A Possible Co-Incidence Causality. The American Journal of Case Reports 23:e936257
Ianache I, Calistru PI, Tardei G, Ruta S, Oprea C (2016) Late presentation in HIV-infected injecting drug users - a huge challenge for the Romanian health-care system. Roman J Leg Med 24(2):122–127
Karila L, Megarbane B, Chevillard L, Benturquia N, Laplanche JL, Lejoyeux M (2015) Novel psychoactive substances: a review. Presse Medicale 44(4 Pt 1):383–391
Kumar S, Rao PS, Earla R, Kumar A (2015) Drug-drug interactions between anti-retroviral therapies and drugs of abuse in HIV systems. Expert Opin Drug Metab Toxicol 11(3):343–355
Manzardo C, Del Mar OM, Sued O, Garcia F, Moreno A, Miro JM (2005) Central nervous system opportunistic infections in developed countries in the highly active antiretroviral therapy era. J Neurovirol 11(Suppl 3):72–82
Mărdărescu M, Popa MI, Streinu-Cercel A (2021) HIV/AIDS IN ROMANIA – A SHORT HISTORY AND UPDATE, 2021. Romanian Archives of Microbiology and Immunology 80:305–311
Matinella A, Lanzafame M, Bonometti MA, Gajofatto A, Concia E, Vento S et al (2015) Neurological complications of HIV infection in pre-HAART and HAART era: a retrospective study. J Neurol 262(5):1317–1327
McNamara PH, Coen R, Redmond J, Doherty CP, Bergin C (2017) A High Prevalence Rate of a Positive Screen for Cognitive Impairment in Patients With Human Immunodeficiency Virus Attending an Irish Clinic. Open For Infect Dis 4(1):ofw242
Mhatre R, Govekar S, Bn N, Lakshman LP, Banda RPS et al (2020) Ocular pathology in NeuroAIDS - An autopsy study. Exper Eye Res 198:108148
Mulherkar TH, Gomez DJ, Sandel G, Jain P (2022) Co-Infection and Cancer: Host-Pathogen Interaction between Dendritic Cells and HIV-1, HTLV-1, and Other Oncogenic Viruses. Viruses 14(9)
Nael A, Walavalkar V, Wu W (2016) CD4-Positive T-cell primary central nervous system lymphoma in an HIV positive patient. Am J Clin Pathol 145(2):258–265
Nath A (2010) Human immunodeficiency virus-associated neurocognitive disorder: pathophysiology in relation to drug addiction. Ann N Y Acad Sci 1187:122–128
Nils von H (2016) Potential Drug Interactions Between cART and New Psychoactive Substances. J Antivir Antiretro 8(1948–5964):1–5
Okurut S, Boulware DR, Olobo J, Meya DB (2020) Landmark clinical observations and immunopathogenesis pathways linked to HIV and Cryptococcus fatal central nervous system co-infection. Mycoses 63(8):840–853
Ondounda M, Ilozue C, Magne C (2016) Cerebro-meningeal infections in HIV-infected patients: a study of 116 cases in Libreville. Gabon African Health Sciences 16(2):603–610
Oprea C, Ianache I, Ionescu P, Ceausu E, Calistru P (2017) Malignancies in HIV-infected patients – incidence and predictors of survival in a Romanian health care facility. Romanian Journal of Legal Medicine 25(2):227–234
Oprea C, Ianache I, Calistru PI, Nica M, Ruta S, Smith C et al (2018) Increasing incidence of HIV- associated tuberculosis in Romanian injecting drug users. HIV Med 19(5):316–323
Parsons TD, Tucker KA, Hall CD, Robertson WT, Eron JJ, Fried MW et al (2006) Neurocognitive functioning and HAART in HIV and hepatitis C virus co-infection. AIDS 20:1591–1595
Post MJ, Thurnher MM, Clifford DB, Nath A, Gonzalez RG, Gupta RK et al (2013a) CNS-immune reconstitution inflammatory syndrome in the setting of HIV infection, part 1: overview and discussion of progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome and cryptococcal-immune reconstitution inflammatory syndrome. AJNR Am J Neuroradiol 34(7):1297–1307
Post MJ, Thurnher MM, Clifford DB, Nath A, Gonzalez RG, Gupta RK et al (2013b) CNS-immune reconstitution inflammatory syndrome in the setting of HIV infection, part 2: discussion of neuro-immune reconstitution inflammatory syndrome with and without other pathogens. AJNR Am J Neuroradiol 34(7):1308–1318
Proceedings of The 8th Romanian National HIV/AIDS Congress and The 3rd Central European HIV Forum : Sibiu, Romania. (2016) BMC Infect Dis 16 Suppl 3:290
Sanchez AB, Varano GP, de Rozieres CM, Maung R, Catalan IC, Dowling CC et al (2015) Antiretrovirals, Methamphetamine, and HIV-1 Envelope Protein gp120 Compromise Neuronal Energy Homeostasis in Association with Various Degrees of Synaptic and Neuritic Damage. Antimicrob Agents Chemother 60(1):168–179
Sheybani F, van de Beek D, Brouwer MC (2021) Suspected Central Nervous System Infections in HIV-Infected Adults. Frontiers in Neurology 12
Tan K, Roda R, Ostrow L, McArthur J, Nath A (2009) PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology 72(17):1458–1464
Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U et al (2007) Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 45(2):174–182
Valcour V, Yee P, Williams AE, Shiramizu B, Watters M, Selnes O et al (2006) Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection–The Hawaii Aging with HIV Cohort. J Neurovirol 12(5):387–391
Venkataramana A, Pardo CA, McArthur JC, Kerr DA, Irani DN, Griffin JW et al (2006) Immune reconstitution inflammatory syndrome in the CNS of HIV-infected patients. Neurology 67(3):383–388
von Wyl V, Klimkait T, Yerly S, Nicca D, Furrer H, Cavassini M et al (2013) Adherence as a predictor of the development of class-specific resistance mutations: the Swiss HIV Cohort Study. PLoS ONE 8(10):e77691
Wright EJ (2014) Neuro OIs: developed and developing countries. Curr Opin HIV AIDS 9(6):539–544
Zeng YM, Li Y, He XQ, Huang YQ, Liu M, Yuan J et al (2020) A study for precision diagnosing and treatment strategies in difficult-to-treat AIDS cases and HIV-infected patients with highly fatal or highly disabling opportunistic infections: Study protocol for antiretroviral therapy timing in AIDS patients with toxoplasma encephalitis. Medicine 99(29):e21141
Acknowledgements
Luminita Ene, Roxana Radoi, Eugenia Ungureanu, Erscoiu Simona, Ionut Popa, Dan Duiculescu, treating physicians.
Author information
Authors and Affiliations
Contributions
Cristiana Oprea: project administration, Cristiana Oprea, and Irina Ianache: conceptualization and methodology design, data curation, and writing the paper, Sorina Vasile, Cristiana Costescu: data curation, Gratiela Tardei, Manuela Nica: laboratory analysis and validation, Anya Umlauf: statistical analysis, revision, and validation, Cristian Achim: supervision and validation.
Corresponding author
Ethics declarations
Ethical approval
Approved by the local Ethical committee.
Inform consent
All the patients signed informed consent at admission in the hospital.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oprea, C., Ianache, I., Vasile, S. et al. Brain opportunistic infections and tumors in people living with HIV — still a challenge in efficient antiretroviral therapy era. J. Neurovirol. 29, 297–307 (2023). https://doi.org/10.1007/s13365-023-01135-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-023-01135-1