Abstract
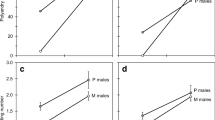
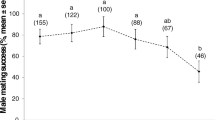
During insect copulation, males typically transfer to females complex ejaculates that contain more than just sperm. These fluids can have a variety of effects on females (e.g., they can supply energy and nutrients, increase egg production and egg laying, inhibit female re-mating, and reduce female longevity). Although these effects of seminal fluid on females are often volume-dependent, and despite the several functions of seminal fluids, few studies have investigated the effects of mating duration on female reproductive traits. We therefore controlled mating duration and investigated the effects of seminal fluid on reproductive traits (lifetime fecundity, oviposition time, and oviposition rate) and longevity of females of the seed bug Togo hemipterus (Scott) (Heteroptera: Lygaeidae). All mated females laid eggs, even after a short mating duration (10 min). After a short mating duration, however, only approximately half of the females laid fertilized eggs, possibly because they did not receive sufficient sperm to fertilize all their eggs. These results showed that female egg laying was induced by such physical stimuli as penis insertion and stretch reception in the bursa copulatrix, or by male seminal products other than sperm. Males must continue ejaculation for 20 min or more to ensure fertilization of the female’s eggs. Although males ejaculated all their sperm within 20 min, a single insemination did not fertilize all of a female’s eggs throughout her lifetime. Females that copulated once laid eggs for approximately 40 days, but laid fertilized eggs for approximately the first 20 days only; we believe the sperm were depleted or became inactive. The positive correlation between mating duration and lifetime fecundity (including both fertilized and unfertilized eggs) and rate of oviposition (the number of eggs laid per day) indicated that the seminal substances stimulated female egg production and/or egg laying, and were transferred to females in a time-dependent manner. Males manipulated females by use of seminal substances. Mating duration did not affect female longevity. T. hemipterus males affected female reproductive traits in several ways. These seemingly manipulative substances are likely to be costly to females because they laid unfertilized eggs when sperm were depleted or became inactive.




Similar content being viewed by others
References
Arnqvist G, Danielsson I (1999) Postmating sexual selection: the effects of male body size and recovery period on paternity and egg production rate in a water strider. Behav Ecol 10:358–365. doi:10.1093/beheco/10.4.358
Arnqvist G, Rowe L (2005) Sexual conflict. Princeton University Press, Princeton
Chapman T (2001) Seminal fluid-mediated fitness traits in Drosophila. Heredity 87:511–521. doi:10.1046/j.1365-2540.2001.00961.x
Chapman T, Davies SJ (2004) Functions and analysis of the seminal fluid proteins of male Drosophila melanogaster fruit flies. Peptides 25:1477–1490. doi:10.1016/j.peptides.2003.10.023
Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L (1995) Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373:241–244. doi:10.1038/373241a0
Chen PS (1984) The functional morphology and biochemistry of insect male accessory glands and their secretions. Ann Rev Entomol 29:233–255. doi:10.1146/annurev.en.29.010184.001313
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton
Fjerdingstad EJ, Boomsma JJ (1998) Multiple mating increases the sperm stores of Atta colombica leafcutter ant queens. Behav Ecol Sociobiol 42:257–261. doi:10.1007/s002650050437
García-González F (2004) Infertile matings and sperm competition: the effect of ‘‘nonsperm representation’’ on intraspecific variation in sperm precedence patterns. Am Nat 164:457–472
Himuro C (2009) Evolutionary ecology research about sexual conflict over reproduction in the seed bug Togo hemipterus (Heteroptera: Lygaeidae). PhD Dissertation, University of Kyoto, Kyoto, Japan (in Japanese)
Himuro C, Fujisaki K (2008) Males of the seed bug Togo hemipterus (Heteroptera: Lygaeidae) use accessory gland substances to inhibit remating by females. J Insect Physiol 54:1538–1542. doi:10.1016/j.jinsphys.2008.09.002
Himuro C, Fujisaki K (2010) Mating experience weakens starvation tolerance in the seed bug Togo hemipterus (Heteroptera: Lygaeidae). Physiol Entomol 35:128–133. doi:10.1111/j.1365-3032.2009.00719.x
Himuro C, Fujisaki K (2012) The effects of male harassment on mating duration in the seed bug, Togo hemipterus. Entomol Exp Appl 142:53–59. doi:10.1111/j.1570-7458.2011.01199.x
Kubli E (2003) Sex-peptides: seminal peptides of the Drosophila male. Cell Mol Life Sci 60:1689–1704. doi:10.1007/s00018-003-3052
McLain DK, Marsh NB (1990) Male copulatory success: heritability and relationship to mate fecundity in the southern green stinkbug, Nezara viridula (Hemiptera: Pentatomidae). Heredity 64:161–167. doi:10.1038/hdy.1990.20
Oku K, Kitsunezuka K (2011) Effects of male mating interval on spermatophore formation, transfer, and subsequent female receptivity and fecundity in the sorghum plant bug, Stenotus rubrovittatus. Entomol Exp Appl 140:134–138. doi:10.1111/j.1570-7458.2011.01142.x
Parker GA (1978) Searching for mates. In: Krebs JR, Davies NB (eds) Behavioural ecology: an evolutionary approach. Blackwell Scientific Publications, Oxford, pp 214–244
Perry JC, Sirot L, Wigby S (2013) The seminal symphony: how to compose an ejaculate. Trends Ecol Evol 28:414–422. doi:10.1016/j.tree.2013.03.005
Poiani A (2006) Complexity of seminal fluid: a review. Behav Ecol Sociobiol 60:289–310. doi:10.1007/s00265-006-0178-0
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225. doi:10.2307/2409177
SAS Institute Inc (2013) JMP® 11 statistics and graphics guide. SAS Institute Inc., Cary
Sheldon BC (1994) Male phenotype, fertility, and the pursuit of extra-pair copulations by female birds. Proc R Soc London Ser B 257:25–30. doi:10.1098/rspb.1994.0089
Simmons LW (2001) Sperm competition and its evolutionary consequences in the insects. Princeton University Press, Princeton
Simmons LW, Siva-Jothy MJ (1998) Sperm competition in insects: mechanisms and the potential for selection. In: Birkhead TR, Moller AP (eds) Sperm competition and sexual selection. Academic Press, London, pp 341–434
Tomokuni M, Yasunaga T, Takai M, Yamashita I, Kawamra M, Kawasawa T (1993) A field guide to Japanese bugs—terrestrial heteropterans. Zenkoku Noson Kyoiku Kyokai Publishing Co. Ltd, Tokyo (in Japanese)
Tram U, Wolfner MF (1999) Male seminal proteins are essential for sperm storage in Drosophila melanogaster. Genetics 153:837–844
Wolfner MF (1997) Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochem Molec Biol 27:179–192. doi:10.1016/S0965-1748(96)00084-7
Acknowledgments
We thank Dr T. Nishida and the members of the Laboratory of Insect Ecology, Kyoto University, for their valuable advice and discussions regarding these experiments. This work was supported in part by a Grant-in-Aid for JSPS Fellows (no. 24-4740) and the twenty first century COE Program for Innovative Food and Environmental Studies Pioneered by Entomomimetic Sciences from the Ministry of Education, Culture, Sports, Science and Technology, Japan (JSPS Grant-in-Aid no. 19-54183).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Himuro, C., Fujisaki, K. Effects of mating duration on female reproductive traits of the seed bug Togo hemipterus (Heteroptera: Lygaeidae). Appl Entomol Zool 50, 491–496 (2015). https://doi.org/10.1007/s13355-015-0357-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-015-0357-4