Abstract
Posaconazole (PSC) is a triazole group anti-fungal agent with the widest spectrum. Although there is no commercially available ocular dosage form, its diluted oral suspension preparation (Noxafil®) is used as off-label in topical treatment of severe keratitis and sclerokeratitis in the clinic. However, ocular bioavailability of PSC suspension form is extremely low due to its highly lipophilic character. Thus, there is a clinical need to improve its ocular bioavailability and to develop novel delivery system for the treatment of ocular fungal infections. Herein, we studied ex vivo permeation, penetration, anti-fungal activity, and Hen’s Egg Test-Chorioallantoic Membrane (HET-CAM) toxicity tests in order to assess ocular targeting of PSC micelles, which were optimized in our previous study. The results indicated that micellar carrier system increased the permeability of PSC to eye tissues. Micelles showed higher affinity to ocular tissues than that of commercial oral suspension of PSC (Noxafil®). In vitro anti-fungal activity data also confirmed the efficacy of PSC loaded micellar formulations against Candida. albicans strains. The relative safety of the optimized micelles on the ocular tissue was shown with the HET-CAM toxicity test. In conclusion, micellar systems could be a promising strategy for the effective and safe delivery of PSC in the treatment of ocular fungal infections.
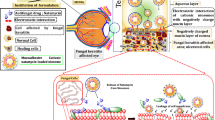
Graphical abstract





Similar content being viewed by others
Availability of data and materials
The datasets generated or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BCS:
-
Biopharmaceutics Classification System
- CMC:
-
Critical micelle concentration
- Deff:
-
Diffusion coefficients
- F127:
-
Pluronic F127
- F68:
-
Pluronic F68
- HET-CAM:
-
Hen’s Egg Test-Chorioallantoic Membrane (HET-CAM)
- HLB:
-
Hydrophilic/lipophilic balance
- HPLC:
-
High-pressure liquid chromatography
- HPLC:
-
International unit
- JSS:
-
Steady-state flux
- MIC:
-
Minimum inhibitor concentration
- Papp:
-
Permeability coefficient
- PEG:
-
Polyethylene glycol
- P-gp:
-
P-glycoprotein
- PSC:
-
Posaconazole
- SD:
-
Standard deviation
- TJs:
-
Tight junctions
- TPGS:
-
D-a-Tocopheryl polyethylene glycol 1000 succinate
References
Özsoy Y, Güngör S, Kahraman E, Durgun ME. Polymeric micelles as a novel carrier for ocular drug delivery. Nanoarchitectonics Biomed., Elsevier; 2019, p. 85–117. https://doi.org/10.1016/B978-0-12-816200-2.00005-0.
Durgun ME, Güngör S, Özsoy Y. Micelles: Promising Ocular Drug Carriers for Anterior and Posterior Segment Diseases. J Ocul Pharmacol Ther 2020;36:jop.2019.0109. https://doi.org/10.1089/jop.2019.0109.
Kanoujia J, Kushwaha PS, Saraf SA. Evaluation of gatifloxacin pluronic micelles and development of its formulation for ocular delivery. Drug Deliv Transl Res. 2014;4:334–43. https://doi.org/10.1007/s13346-014-0194-y.
Al Khateb K, Ozhmukhametova EK, Mussin MN, Seilkhanov SK, Rakhypbekov TK, Lau WM, et al. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int J Pharm. 2016;502:70–9. https://doi.org/10.1016/j.ijpharm.2016.02.027.
Zhou T, Zhu L, Xia H, He J, Liu S, He S, et al. Micelle carriers based on macrogol 15 hydroxystearate for ocular delivery of terbinafine hydrochloride: in vitro characterization and in vivo permeation. Eur J Pharm Sci. 2017;109:288–96. https://doi.org/10.1016/j.ejps.2017.08.020.
Luschmann C, Tessmar J, Schoeberl S, Strau O, Luschmann K, Goepferich A. Self-assembling colloidal system for the ocular administration of cyclosporine A. Cornea. 2014;33:77–81. https://doi.org/10.1097/ICO.0b013e3182a7f3bf.
Cholkar K, Patel A, Dutt Vadlapudi A, K. Mitra A. Novel Nanomicellar Formulation Approaches for Anterior and Posterior Segment Ocular Drug Delivery. Recent Patents Nanomedicinee 2012;2:82–95. https://doi.org/10.2174/1877912311202020082.
Mandal A, Bisht R, Rupenthal ID, Mitra AK. Polymeric micelles for ocular drug delivery: from structural frameworks to recent preclinical studies. J Control Release. 2017;248:96–116. https://doi.org/10.1016/j.jconrel.2017.01.012.
Mandal A, Gote V, Pal D, Ogundele A, Mitra AK. Ocular pharmacokinetics of a topical ophthalmic nanomicellar solution of cyclosporine (Cequa®) for dry eye disease. Pharm Res. 2019;36:36. https://doi.org/10.1007/s11095-018-2556-5.
Smyth-Medina R, Johnston J, Devries DK, Jasper A, Kannarr SR, Schechter BA, et al. Effect of OTX-101, a novel nanomicellar formulation of cyclosporine A, on conjunctival staining in patients with keratoconjunctivitis sicca: a pooled analysis of phase 2b/3 and 3 clinical trials. J Ocul Pharmacol Ther. 2019;35:388–94. https://doi.org/10.1089/jop.2018.0154.
Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. 2017;117:14–28. https://doi.org/10.1016/j.ejpb.2017.03.006.
Walimbe T, Chelerkar V, Bhagat P, Joshi A, Raut A. Effect of benzalkonium chloride-free latanoprost ophthalmic solution on ocular surface in patients with glaucoma. Clin Ophthalmol 2016:821. https://doi.org/10.2147/OPTH.S102976.
Ibrahim SS. The role of surface active agents in ophthalmic drug delivery: a comprehensive review. J Pharm Sci. 2019;108:1923–33. https://doi.org/10.1016/j.xphs.2019.01.016.
Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm. 2002;28:353–69. https://doi.org/10.1081/DDC-120002997.
Jiao J. Polyoxyethylated nonionic surfactants and their applications in topical ocular drug delivery. Adv Drug Deliv Rev. 2008;60:1663–73. https://doi.org/10.1016/j.addr.2008.09.002.
Grimaudo MA, Pescina S, Padula C, Santi P, Concheiro A, Alvarez-Lorenzo C, et al. Poloxamer 407/TPGS mixed micelles as promising carriers for cyclosporine ocular delivery. Mol Pharm. 2018;15:571–84. https://doi.org/10.1021/acs.molpharmaceut.7b00939.
Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS PharmSciTech. 2009;10:808. https://doi.org/10.1208/s12249-009-9268-4.
Eljarrat-Binstock E, Pe’er J, Domb AJ. New Techniques for Drug Delivery to the Posterior Eye Segment. Pharm Res 2010;27:530–43. https://doi.org/10.1007/s11095-009-0042-9.
Guo Y, Luo J, Tan S, Otieno BO, Zhang Z. The applications of vitamin e TPGS in drug delivery. Eur J Pharm Sci. 2013;49:175–86. https://doi.org/10.1016/j.ejps.2013.02.006.
Kandekar SG, del Río-Sancho S, Lapteva M, Kalia YN. Selective delivery of adapalene to the human hair follicle under finite dose conditions using polymeric micelle nanocarriers. Nanoscale. 2018;10:1099–110. https://doi.org/10.1039/C7NR07706H.
Zhang Z, Tan S, Feng SS. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33:4889–906. https://doi.org/10.1016/j.biomaterials.2012.03.046.
Ke WT, Lin SY, Ho HO, Sheu MT. Physical characterizations of microemulsion systems using tocopheryl polyethylene glycol 1000 succinate (TPGS) as a surfactant for the oral delivery of protein drugs. J Control Release. 2005;102:489–507. https://doi.org/10.1016/j.jconrel.2004.10.030.
Tan S, Zou C, Zhang W, Yin M, Gao X, Tang Q. Recent developments ind-a-tocopheryl polyethylene glycol-succinate-based nanomedicine for cancer therapy. Drug Deliv. 2017;24:1831–42. https://doi.org/10.1080/10717544.2017.1406561.
Rangel-Yagui CO, Pessoa-Jr A, Tavares LC. Micellar solubilization of drugs. J Pharm Pharm Sci. 2005;8:147–63.
Andes D. Optimizing antifungal choice and administration. Curr Med Res Opin. 2013;29:13–8. https://doi.org/10.1185/03007995.2012.761135.
Hens B, Bermejo M, Tsume Y, Gonzalez-Alvarez I, Ruan H, Matsui K, et al. Evaluation and optimized selection of supersaturating drug delivery systems of posaconazole (BCS class 2b) in the gastrointestinal simulator (GIS): an in vitro-in silico-in vivo approach. Eur J Pharm Sci. 2018;115:258–69. https://doi.org/10.1016/j.ejps.2018.01.039.
Arendrup MC, Cuenca-Estrella M, Donnelly JP, Hope W, Lass-Flörl C, Rodriguez-Tudela JL. EUCAST technical note on posaconazole*. Clin Microbiol Infect. 2011;17:E16–7. https://doi.org/10.1111/j.1469-0691.2011.03646.x.
Sponsel WE, Graybill JR, Nevarez HL, Dang D. Ocular and systemic posaconazole(SCH-56592) treatment of invasive Fusarium solani keratitis and endophthalmitis. Br J Ophthalmol. 2002;86:829–30. https://doi.org/10.1136/bjo.86.7.829-a.
Amiel H, Chohan AB, Snibson GR, Vajpayee R. Atypical fungal sclerokeratitis. Cornea. 2008;27:382–3. https://doi.org/10.1097/ICO.0b013e31815e9298.
Lakhani P, Patil A, Majumdar S. Challenges in the polyene- and azole-based pharmacotherapy of ocular fungal infections. J Ocul Pharmacol Ther. 2019;35:6–22. https://doi.org/10.1089/jop.2018.0089.
Yun T, Shuang L, Yongqiang Z. A kind of preparation method of posaconazole lyophilized injectable powder. CN201510270331.1A, 2017.
Pescina S, Lucca L, Govoni P, Padula C, Favero E, Cantù L, et al. Ex Vivo Conjunctival retention and transconjunctival transport of poorly soluble drugs using polymeric micelles. Pharmaceutics. 2019;11:476. https://doi.org/10.3390/pharmaceutics11090476.
Durgun ME, Kahraman E, Güngör S, Özsoy Y. Optimization and characterization of aqueous micellar formulations for ocular delivery of an antifungal drug. Posaconazole Curr Pharm Des. 2020;26:1543–55. https://doi.org/10.2174/1381612826666200313172207.
Epstein SP, Ahdoot M, Marcus E, Asbell PA. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. J Ocul Pharmacol Ther. 2009;25:113–9. https://doi.org/10.1089/jop.2008.0098.
Jaiswal M, Kumar M, Pathak K. Zero order delivery of itraconazole via polymeric micelles incorporated in situ ocular gel for the management of fungal keratitis. Colloids Surfaces B Biointerfaces. 2015;130:23–30. https://doi.org/10.1016/j.colsurfb.2015.03.059.
Dutescu RM, Panfil C, Schrage N. Osmolarity of prevalent eye drops, side effects, and therapeutic approaches. Cornea. 2015;34:560–6. https://doi.org/10.1097/ICO.0000000000000368.
EEBA. Technical Guidelines for Ocular Tissue (Revision 11) n.d. https://www.eeba.eu/technical-guidelines-for-ocular-tissue-revision-11.html (accessed March 25, 2020).
Dai Y, Zhou R, Liu L, Lu Y, Qi J, Wu W. Liposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): in vitro characterization and improved corneal permeation. Int J Nanomedicine. 2013;8:1921–33. https://doi.org/10.2147/IJN.S44487.
Morrison PWJ, Khutoryanskiy VV. Enhancement in corneal permeability of riboflavin using calcium sequestering compounds. Int J Pharm. 2014;472:56–64. https://doi.org/10.1016/j.ijpharm.2014.06.007.
Moiseev R, Morrison P, Steele F, Khutoryanskiy V. Penetration enhancers in ocular drug delivery. Pharmaceutics. 2019;11:321. https://doi.org/10.3390/pharmaceutics11070321.
Ban J, Zhang Y, Huang X, Deng G, Hou D, Chen Y, et al. Corneal permeation properties of a charged lipid nanoparticle carrier containing dexamethasone. Int J Nanomedicine. 2017;12:1329–39. https://doi.org/10.2147/IJN.S126199.
Clinical and Laboratory Standards Institute. Method for antifungal disk diffusion susceptibility testing of yeasts: approved guideline M44-A. Clin Lab Stand Inst 2009.
Brown S, Traczewski M. Quality control limits for posaconazole disk susceptibility tests on Mueller-Hinton agar with glucose and methylene blue. J Clin Microbiol. 2007;45:222–3. https://doi.org/10.1128/JCM.01732-06.
Luepke NP, Kemper FH. The HET-CAM test: an alternative to the Draize eye test. Food Chem Toxicol. 1986;24:495–6. https://doi.org/10.1016/0278-6915(86)90099-2.
ICCVAM. Recommended Test Method Protocol: Hen’s Egg Test – Chorioallantoic Membrane (HET-CAM) Test Method n.d. https://ntp.niehs.nih.gov/iccvam/docs/protocols/ivocular-hetcam.pdf.
Pepic I, Lovric J, Filipovic-Grcic J. Polymeric micelles in ocular drug delivery: rationale, strategies and challenges. Chem Biochem Eng Q. 2012;26:365.
Cholkar K, Gunda S, Earla R, Pal D, Mitra AK. Nanomicellar topical aqueous drop formulation of rapamycin for back-of-the-eye delivery. AAPS PharmSciTech. 2015;16:610–22. https://doi.org/10.1208/s12249-014-0244-2.
Li M, Qiao N, Wang K. Influence of sodium lauryl sulfate and Tween 80 on carbamazepine-nicotinamide cocrystal solubility and dissolution behaviour. Pharmaceutics. 2013;5:508–24. https://doi.org/10.3390/pharmaceutics5040508.
Sulek MW, Wasilewski T, Kurzydłowski KJ. The effect of concentration on lubricating properties of aqueous solutions of sodium lauryl sulfate and ethoxylated sodium lauryl sulfate. Tribol Lett. 2010;40:337–45. https://doi.org/10.1007/s11249-010-9668-3.
Simroth‐Loch C, Weitschies W, Wilson C. Ophthalmic Dosage Forms. In: Fotaki N, Klein S, editors. Vitr. Drug Release Test. Spec. Dos. Forms. 1st ed., John Wiley & Sons, Ltd; 2019, p. 235–52.
Mazyed EA, Abdelaziz AE. Fabrication of transgelosomes for enhancing the ocular delivery of acetazolamide: statistical optimization, in vitro characterization, and in vivo study. Pharmaceutics. 2020;12:465. https://doi.org/10.3390/pharmaceutics12050465.
Sharpe S, Sequeira J, Harris D, Shashank M. Antifungal Composition with enhanced Bioavailability. US 8.263,600 B2, 2012.
Sharma PK, Chauhan MK. Optimization and evaluation of encapsulated brimonidine tartrate-loaded nanoparticles incorporation in situ gel for efficient intraocular pressure reduction. J Sol-Gel Sci Technol. 2020;95:190–201. https://doi.org/10.1007/s10971-020-05305-z.
Alkholief M, Albasit H, Alhowyan A, Alshehri S, Raish M, Abul Kalam M, et al. Employing a PLGA-TPGS based nanoparticle to improve the ocular delivery of Acyclovir. Saudi Pharm J. 2019;27:293–302. https://doi.org/10.1016/j.jsps.2018.11.011.
Durairaj C. Ocular Pharmacokinetics. In: Whitcup S, Azar D, editors. Pharmacol. Ther. Ocul. Dis., vol. 242. 1st ed., Springer, Cham; 2016, p. 31–55. https://doi.org/10.1007/164_2016_32.
Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–5. https://doi.org/10.1016/j.addr.2006.07.027.
Trivedi R, Kompella UB. Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine. 2010;5:485–505. https://doi.org/10.2217/nnm.10.10.
Tavares Luiz M, Delello Di Filippo L, Carolina Alves R, Sousa Araújo VH, Lobato Duarte J, Maldonado Marchetti J, et al. The use of TPGS in drug delivery systems to overcome biological barriers. Eur Polym J 2021;142:110129. https://doi.org/10.1016/j.eurpolymj.2020.110129.
Mortensen K, Batsberg W, Hvidt S. Effects of PEO−PPO diblock impurities on the cubic structure of aqueous PEO−PPO−PEO pluronics micelles: fcc and bcc ordered structures in F127. Macromolecules. 2008;41:1720–7. https://doi.org/10.1021/ma702269c.
Fule R, Amin P. Hot melt extruded amorphous solid dispersion of posaconazole with improved bioavailability: investigating drug-polymer miscibility with advanced characterisation. Biomed Res Int. 2014;2014:1–16. https://doi.org/10.1155/2014/146781.
Schoenwald R, Huang H. Corneal penetration behavior of β-blocking agents I: physicochemical factors. J Pharm Sci. 1983;72:1266–72. https://doi.org/10.1002/jps.2600721108.
Goyal R, Macri L, Kohn J. Formulation strategy for the delivery of cyclosporine A: comparison of two polymeric nanospheres. Sci Rep. 2015;5:13065. https://doi.org/10.1038/srep13065.
Carrillo-Muñoz AJ, Quindós G, Ruesga M, Alonso R, del Valle O, Hernández-Molina JM, et al. Antifungal activity of posaconazole compared with fluconazole and amphotericin B against yeasts from oropharyngeal candidiasis and other infections. J Antimicrob Chemother. 2005;55:317–9. https://doi.org/10.1093/jac/dki022.
Oude Lashof AML, Rothova A, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, et al. Ocular manifestations of candidemia. Clin Infect Dis. 2011;53:262–8. https://doi.org/10.1093/cid/cir355.
Regan F, Chapman J, Sullivan T. Biological Methods for Characterisation of Nano-Anti-Microbial Materials. In: Regan F, Chapman J, Sullivan T, editors. Nanoparticles Anti-Microbial Mater. Use Characterisation. 1st ed., The Royal Society of Chemistry; 2012, p. 153–92.
Alambiaga-Caravaca AM, Calatayud-Pascual MA, Rodilla V, Concheiro A, López-Castellano A, Alvarez-Lorenzo C. Micelles of progesterone for topical eye administration: interspecies and intertissues differences in ex vivo ocular permeability. Pharmaceutics. 2020;12:702. https://doi.org/10.3390/pharmaceutics12080702.
Wilson SL, Ahearne M, Hopkinson A. An overview of current techniques for ocular toxicity testing. Toxicology. 2015;327:32–46. https://doi.org/10.1016/j.tox.2014.11.003.
Scheel J, Kleber M, Kreutz J, Lehringer E, Mehling A, Reisinger K, et al. Eye irritation potential: usefulness of the HET-CAM under the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). Regul Toxicol Pharmacol. 2011;59:471–92. https://doi.org/10.1016/j.yrtph.2011.02.003.
Acknowledgements
The authors are grateful to BASF-Turkey for their support by Kolliphor TPGS, Pluronic F127 and Pluronic F68. Also, they thank Çekmece slaughterhouse and meat products for their support by isolated eyes supply.
Funding
The present work was supported by the Research Fund of Istanbul University (Project No: 20958).
Author information
Authors and Affiliations
Contributions
The submitted work is based primarily on MED’s doctoral dissertation. Encouragement to investigate PSC-loaded micelles as potential nanocarriers for ocular application, supervision of the project and the findings of this work were provided by YÖ. Methodology and performation of the experiments [MED] except for the microbiological studies: [MH]. Literature search and implementing the review of the literature: [MED]; writing—the draft preparation: [MED]; review and editing: [MED, EK, SG and YÖ]; funding acquisition: [YÖ]; resources: [Research Fund of Istanbul University]. The first draft of the manuscript was written by [MED] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate:
No ethics approval was required for the experiments performed in the scope of this manuscript. We declare that all the experiments comply with the current laws. The research involved no animal or human participants.
Consent for publication:
All the authors agreed with the content and gave explicit consent to submit the work. We obtained the required consent from the responsible authority, the Graduate School of Health Science at Istanbul University. All the authors approved the version to be published..
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Durgun, M.E., Kahraman, E., Hacıoğlu, M. et al. Posaconazole micelles for ocular delivery: in vitro permeation, ocular irritation and antifungal activity studies. Drug Deliv. and Transl. Res. 12, 662–675 (2022). https://doi.org/10.1007/s13346-021-00974-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-00974-x