Abstract
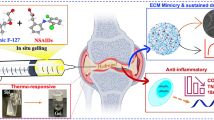
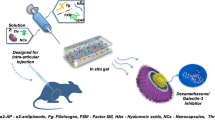
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by joint synovial inflammation, as well as cartilage and bone tissue destruction. Current strategies for the treatment of RA can reduce joint inflammation, but the treatment options still represent stability concerns since they are not sufficient and present a fast clearing. Thus, several drug delivery systems (DDS) have been advanced to tackle this limitation. Injectable gellan gum (GG) hydrogels, reduced by physical crosslinking methods, also being proposed as DDS, but this kind of crosslinking can produce hydrogels that become weaker in physiological conditions. Nevertheless, enzymatic crosslinking emerged as an alternative to increase mechanical strength, which can be adjusted by the degree of enzymatic crosslinking. In this study, tyramine-modified gellan gum (Ty-GG) hydrogels were developed via horseradish peroxidase (HRP) crosslinking; and betamethasone was encapsulated within, to increase the specificity and safety in the treatment of patients with RA. Physicochemical results showed that it was possible to modify GG with tyramine, with a degree of substitution of approximately 30%. They showed high mechanical strength and resistance, presenting a controlled betamethasone release profile over time. Ty-GG hydrogels also exhibited no cytotoxic effects and do not negatively affected the metabolic activity and proliferation of chondrogenic primary cells. Furthermore, the main goal was achieved since betamethasone-loaded Ty-GG hydrogels demonstrated to have a more effective therapeutic effect when compared with the administration of betamethasone alone. Therefore, the developed Ty-GG hydrogels represent a promising DDS and a reliable alternative to traditional treatments in patients with RA.









Similar content being viewed by others
References
Wang M, Wei J, Li H, Ouyang X, Sun X, Tang Y, et al. Leptin upregulates peripheral CD4+ CXCR5+ ICOS+ T cells via increased IL-6 in rheumatoid arthritis patients. J Interf Cytokine Res. 2018;38(2):86–92.
Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6(1):1–14.
Pirmardvand Chegini S, Varshosaz J, Taymouri S. Recent approaches for targeted drug delivery in rheumatoid arthritis diagnosis and treatment. Artif Cells Nanomed Biotechnol. 2018;46(sup2):502–14.
Andersen NS, Cadahia JP, Previtali V, Bondebjerg J, Hansen CA, Hansen AE, et al. Methotrexate prodrugs sensitive to reactive oxygen species for the improved treatment of rheumatoid arthritis. Eur J Med Chem. 2018;156:738–46.
Gouveia VM, Lima SCA, Nunes C, Reis S. Non-biologic nanodelivery therapies for rheumatoid arthritis. J Biomed Nanotechnol. 2015;11(10):1701–21.
Yang M, Feng X, Ding J, Chang F, Chen X. Nanotherapeutics relieve rheumatoid arthritis. J Control Release. 2017;252:108–24.
Prosperi D, Colombo M, Zanoni I, Granucci F, editors. Drug nanocarriers to treat autoimmunity and chronic inflammatory diseases. Semin Immunol; 2017: Elsevier.
Feng X, Chen Y. Drug delivery targets and systems for targeted treatment of rheumatoid arthritis. J Drug Target. 2018;26(10):845–57.
Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49(8):1993–2007.
Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12):1–17.
Xu Y, Li Y, Chen Q, Fu L, Tao L, Wei Y. Injectable and self-healing chitosan hydrogel based on imine bonds: design and therapeutic applications. Int J Mol Sci. 2018;19(8):2198.
Yang J-A, Yeom J, Hwang BW, Hoffman AS, Hahn SK. In situ-forming injectable hydrogels for regenerative medicine. Prog Polym Sci. 2014;39(12):1973–86.
Harding NE, Patel YN, Coleman RJ. Organization of genes required for gellan polysaccharide biosynthesis in Sphingomonas elodea ATCC 31461. J Ind Microbiol Biotechnol. 2004;31(2):70–82.
Shin H, Olsen BD, Khademhosseini A. Gellan gum microgel-reinforced cell-laden gelatin hydrogels. J Mater Chem B. 2014;2(17):2508–16.
Hu W, Wang Z, Xiao Y, Zhang S, Wang J. Advances in crosslinking strategies of biomedical hydrogels. Biomater Sci. 2019;7(3):843–55.
Li X, Sun Q, Li Q, Kawazoe N, Chen G. Functional hydrogels with tunable structures and properties for tissue engineering applications. Front Chem. 2018;6:499.
Parhi R. Cross-linked hydrogel for pharmaceutical applications: a review. Adv Pharm Bull. 2017;7(4):515–30.
Akhtar MF, Hanif M, Ranjha NM. Methods of synthesis of hydrogels… a review. Saudi Pharm J. 2016;24(5):554–9.
Khanmohammadi M, Dastjerdi MB, Ai A, Ahmadi A, Godarzi A, Rahimi A, et al. Horseradish peroxidase-catalyzed hydrogelation for biomedical applications. Biomater Sci. 2018;6(6):1286–98.
Lee F, Bae KH, Kurisawa M. Injectable hydrogel systems crosslinked by horseradish peroxidase. Biomed Mater. 2015;11(1):014101.
Ribeiro VP. Multifunctional silk fibroin-based constructs for tissue engineering and regenerative medicine applications. [Doctoral dissertation]. [Guimarães, PT]: Minho University; 2018.
Ribeiro VP, da Silva MA, Maia FR, Canadas RF, Costa JB, Oliveira AL, et al. Combinatory approach for developing silk fibroin scaffolds for cartilage regeneration. Acta Biomater. 2018;72:167–81.
Bae JW, Choi JH, Lee Y, Park KD. Horseradish peroxidase-catalysed in situ-forming hydrogels for tissue-engineering applications. J Tissue Eng Regen Med. 2015;9(11):1225–32.
Oryan A, Kamali A, Moshiri A, Baharvand H, Daemi H. Chemical crosslinking of biopolymeric scaffolds: current knowledge and future directions of crosslinked engineered bone scaffolds. Int J Biol Macromol. 2018;107:678–88.
Prodanovic O, Spasojevic D, Prokopijevic M, Radotic K, Markovic N, Blazic M, et al. Tyramine modified alginates via periodate oxidation for peroxidase induced hydrogel formation and immobilization. React Funct Polym. 2015;93:77–83.
Coutinho DF, Sant SV, Shin H, Oliveira JT, Gomes ME, Neves NM, et al. Modified gellan gum hydrogels with tunable physical and mechanical properties. Biomaterials. 2010;31(29):7494–502.
Reys LL, Silva SS, Soares da Costa D, Oliveira NM, Mano Jo F, Reis RL, et al. Fucoidan hydrogels photo-cross-linked with visible radiation as matrices for cell culture. ACS Biomater Sci Eng. 2016;2(7):1151–61.
Kim K, Park S, Yang J-A, Jeon J-H, Bhang S, Kim B-S, et al. Injectable hyaluronic acid–tyramine hydrogels for the treatment of rheumatoid arthritis. Acta Biomater. 2011;7(2):666–74.
Darr A, Calabro A. Synthesis and characterization of tyramine-based hyaluronan hydrogels. J Mater Sci Mater Med. 2009;20(1):33–44.
Loebel C, D’Este M, Alini M, Zenobi-Wong M, Eglin D. Precise tailoring of tyramine-based hyaluronan hydrogel properties using DMTMM conjugation. Carbohydr Polym. 2015;115:325–33.
Wennink JW, Niederer K, Bochyńska AI, Moreira Teixeira LS, Karperien M, Feijen J et al., editors. Injectable hydrogels by enzymatic co-crosslinking of dextran and hyaluronic acid tyramine conjugates. Macromol Symp; 2011: Wiley Online Library.
Prokopijevic M, Prodanovic O, Spasojevic D, Kovacevic G, Polovic N, Radotic K, et al. Tyramine-modified pectins via periodate oxidation for soybean hull peroxidase induced hydrogel formation and immobilization. Appl Microbiol Biotechnol. 2017;101(6):2281–90.
Jagur-Grodzinski J. Polymeric gels and hydrogels for biomedical and pharmaceutical applications. Polym Adv Technol. 2010;21(1):27–47.
Huang Y, Yu H, Xiao C. pH-sensitive cationic guar gum/poly (acrylic acid) polyelectrolyte hydrogels: swelling and in vitro drug release. Carbohydr Polym. 2007;69(4):774–83.
da Silva LP, Cerqueira MT, Sousa RA, Reis RL, Correlo VM, Marques AP. Engineering cell-adhesive gellan gum spongy-like hydrogels for regenerative medicine purposes. Acta Biomater. 2014;10(11):4787–97.
Flory PJ. Thermodynamics of high polymer solutions. J Chem Phys. 1941;9(8):660.
Jin R, Hiemstra C, Zhong Z, Feijen J. Enzyme-mediated fast in situ formation of hydrogels from dextran–tyramine conjugates. Biomaterials. 2007;28(18):2791–800.
Nguyen QV, Park JH, Lee DS. Injectable polymeric hydrogels for the delivery of therapeutic agents: a review. Eur Polym J. 2015;72:602–19.
Jin R, Lin C, Cao A. Enzyme-mediated fast injectable hydrogels based on chitosan-glycolic acid/tyrosine: preparation, characterization, and chondrocyte culture. Polym Chem. 2013;5. https://doi.org/10.1039/C3PY00864A.
Buitrago-Vásquez M, Ossa-Orozco CP. Degradation, mater uptake, injectability and mechanical strength of injectable bone substitutes composed of silk fibroin and hydroxyapatite nanorods. Rev Fac the Ing. 2018;27(48):49–60.
Nam JG, Hyun K, Ahn KH, Lee SJ. Phase angle of the first normal stress difference in oscillatory shear flow. Korea-Aust Rheol J. 2010;22(4):247–57.
Ngan CL, Basri M, Lye FF, Masoumi HRF, Tripathy M, Karjiban RA, et al. Comparison of process parameter optimization using different designs in nanoemulsion-based formulation for transdermal delivery of fullerene. Int J Nanomedicine. 2014;9:4375.
Cai Z, Zhang H, Wei Y, Wu M, Fu A. Shear-thinning hyaluronan-based fluid hydrogels to modulate viscoelastic properties of osteoarthritis synovial fluids. Biomater Sci. 2019;7(8):3143–57.
Galesso D, Finelli I, Paradossi G, Renier D. Viscoelastic properties and elastic recovery of HYADD® 4 hydrogel compared to crosslinked HA-based commercial viscosupplements. Osteoarthr Cartil. 2012;20:S292.
Lawless BM, Sadeghi H, Temple DK, Dhaliwal H, Espino DM, Hukins DW. Viscoelasticity of articular cartilage: analysing the effect of induced stress and the restraint of bone in a dynamic environment. J Mech Behav Biomed Mater. 2017;75:293–301.
Risbud MV, Bhonde RR. Polyacrylamide-chitosan hydrogels: in vitro biocompatibility and sustained antibiotic release studies. Drug Deliv. 2000;7(2):69–75.
Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12):16071.
Zhang J, Ma PX. Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv Drug Deliv Rev. 2013;65(9):1215–33.
Dreyer SJ, Beckworth WJ. 2 - Commonly used medications in procedures. In: Lennard TA, Walkowski S, Singla AK, Vivian DG, editors. Pain Procedures in Clinical Practice. Third ed. Saint Louis: Hanley & Belfus; 2011. p. 5–12.
Jacobs JWG, Bijlsma JWJ. Chapter 60 - Glucocorticoid therapy. In: Firestein GS, Budd RC, Gabriel SE, IB MI, O'Dell JR, editors. Kelley and Firestein's Textbook of Rheumatology. Tenth ed: Elsevier; 2017. p. 932–57.e5.
Wernecke C, Braun HJ, Dragoo JL. The effect of intra-articular corticosteroids on articular cartilage: a systematic review. Orthop J Sports Med. 2015;3(5):2325967115581163. https://doi.org/10.1177/2325967115581163.
Østergaard M, Halberg P. Intra-articular corticosteroids in arthritic disease. BioDrugs. 1998;9(2):95–103. https://doi.org/10.2165/00063030-199809020-00002.
Silva-Correia J, Oliveira JM, Caridade S, Oliveira JT, Sousa R, Mano J, et al. Gellan gum-based hydrogels for intervertebral disc tissue-engineering applications. J Tissue Eng Regen Med. 2011;5(6):e97–e107.
Khang G, Lee S, Kim H, Silva-Correia J, Gomes ME, Viegas C, et al. Biological evaluation of intervertebral disc cells in different formulations of gellan gum-based hydrogels. J Tissue Eng Regen Med. 2015;9(3):265–75.
Silva-Correia J, Zavan B, Vindigni V, Oliveira MB, Mano J, Pereira H et al. Mechanical performance and biocompatibility study of methacrylated gellan gum hydrogels with potential for nucleus pulposus regeneration. J Tissue Eng Regen Med. 2012;6(2):18.
Kesavan K, Nath G, Pandit J. Preparation and in vitro antibacterial evaluation of gatifloxacin mucoadhesive gellan system. Daru. 2010;18(4):237.
Carmona-Moran C, Zavgorodnya O, Penman A, Kharlampieva E, Bridges S, Hergenrother R, et al. Development of gellan gum containing formulations for transdermal drug delivery: component evaluation and controlled drug release using temperature responsive nanogels. Int J Pharm. 2016;509:465–76. https://doi.org/10.1016/j.ijpharm.2016.05.062.
Norazemi N, Che Rose L, Rose, Mat Amin KA, Suhaimi H, Chan S-Y. Coated gellan gum hydrogel as a drug carrier for colon targeted drug delivery. J Sustain Sci Manag. 2017;2:36–41.
Acknowledgments
I. Oliveira thanks the financial support under the Norte2020 project (“NORTE-08-5369-FSE-000044”), REMIX project (G.A. 778078 — REMIX — H2020-MSCA-RISE-2017), and Gilson Lab, Chonbuk National University, Republic of Korea. The FCT distinction attributed to J. Miguel Oliveira under the Investigator FCT program (IF/01285/2015) is also greatly acknowledged. C. Gonçalves also wish to acknowledge FCT for supporting her research (No. SFRH/BPD/94277/2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The experiments comply with the current laws of the country in which they were performed.
Animal studies
The national and institutional guidelines and certification for the animal experimentation were followed as approved by the Direcção Geral de Alimentação e Veterinária (DGAV) and following the local ethical committee recommendations.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oliveira, I.M., Gonçalves, C., Shin, M.E. et al. Enzymatically crosslinked tyramine-gellan gum hydrogels as drug delivery system for rheumatoid arthritis treatment. Drug Deliv. and Transl. Res. 11, 1288–1300 (2021). https://doi.org/10.1007/s13346-020-00855-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00855-9