Abstract
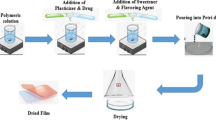
The objective of this work was to design a diclofenac epolamine (DE) flash tablets (FTs) intended to dissolve in the mouth saliva, thereby improving the DE bioavailability and reducing its first-pass liver metabolism. Design-Expert software was used to build a 31.22 full factorial design (12 runs). FTs were fabricated using lyophilization process. The dissolution response was selected to pick the optimized run. The results indicate that the optimized run (R1) showed the fastest drug dissolution (total dissolution in 12 min). The predicted run (Rp) showed a desirability of about 0.93. Differential scanning calorimetry(DSC) analysis results showed a decrease in the drug melting point of the R1 formulation. Fourier–transform infrared spectroscopy (FTIR) showed the compatibility of the drug with other components of formulation, X-ray powder diffraction (XRPD) analysis showed the evolution of the drug physical state from a crystalline to an amorphous form and scanning electron microscopy(SEM) divugled the disappearance of drug crystals in gelatin strands. The results of the pharmacokinetic study performed in 6 human volunteers evidenced an increase in the maximum DE concentration in plasma and, consequently, an increased bioavailability of the FT formulation as compared with a reference formulation(Fr). Concisely, the developed FTs (R1) showed promising results which could be able to enhance oral bioavailability, reduce the therapeutic dose of the drug, and abate of the complications accompanied with conventional dosage forms.

Graphical abstract











Similar content being viewed by others
References
Montenegro-Nicolini M, Morales JO. Overview and future potential of buccal mucoadhesive films as drug delivery systems for biologics. AAPS PharmSciTech. 2017;18(1):3–14.
Chen L, Daoxiao CH, Xinhui ZH, Hong S, Yindi K, Rongyue ZH. Orally fast-dissolving films containing lutein nanocrystals for improved bioavailability: formulation development, in vitro and in vivo evaluation. AAPS PharmSciTech. 2017;18(8):2957–64.
Muñoz H, Castan H, Clares B, Ruiz MA. Obtaining fastdissolving disintegrating tablets with different doses of melatonin. Int J Pharm. 2014;467:84–8.
Hu X, Li Y. Zhang. Preparation and evaluation of orally disintegrating tablets containing taste-masked microcapsules of berberine hydrochloride. AAPS PharmSciTech. 2013;14(1):29–37.
Patravale VB, Date AA, Kulkarni RM. Nanosuspensions: a promising drug delivery strategy. J Pharm Pharmacol. 2004;56:827–40.
Hirani JJ, Rathod DA, Vadalia KR. Orally disintegrating tablets: a review. Trop J Pharm Res. 2009;8(2):161–72.
Alayoubi A, Daihom B, Adhikari H. Development of a taste-masked oral suspension of clindamycin HCl using ion exchange resin Amberlite IRP 69 for use in pediatrics. Drug Dev Ind Pharm. 2016;42(10):1579–89.
Witold B, Ewelina M, Renata J. Orodispersible films and tablets with prednisolone microparticles. Eur J Pharm Sci. 2015;75:81–90.
Battu SK, Repka MA, Majumdar S, Madhusudan RY. Formulation and evaluation of rapidly disintegratingfenoverine tablets: effect of superdisintegrants. Drug Dev Ind Pharm. 2007;33(11):1225–32.
Slavkova M, Breitkreutz J. Orodispersible drug formulations for children and elderly. Eur J Pharm Sci. 2015;75:2–9.
Gustavo F, Peter K, Jörg B. Orodispersible tablets containing taste-masked solid lipid pellets with metformin hydrochloride: influence of process parameters on tablet properties. Eur J Pharm Biopharm. 2018;122:137–45.
Emad BB, Asmaa A, Magdy I. Utility of mannitol and citric acid for enhancing the solubilizing and taste masking properties of β-cyclodextrin: development of fast-dissolving tablets containing extremely bitter drug. J Pharm Innov. 2014;9:309–20.
Battu SK, Repka MA, Majumdar S, Madhusudan RY. Formulation and evaluation of rapidly disintegrating fenoverine tablets: effect of superdisintegrants. Drug Dev Ind Pharm. 2007;33(11):1225–32.
Mutasem MR, Estelle RS, Keith JS. Fast-disintegrating sublingual epinephrine tablets: effect of tablet dimensions on tablet characteristics. Drug Dev Ind Pharm. 2007;33:523–30.
Abdelbary G, Eouani C, Prinderre P, Joachim J, Reynier J, Piccerelle P. Determination of the in vitro disintegration profileof rapidly disintegrating tablets and correlation with oral disintegration. Int J Pharm. 2005;292:29–41.
Patel VF, Liu F, Brown MB. Advances in oral transmucosal drug delivery. J Control Release. 2011;153:106–16.
McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclo-oxygenase. JAMA Oncol. 2006;296(13):1633–44.
McGettigan P, Henry D. Cardiovascular risk with nonsteroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med. 2011;8(9):e1001098.
Scheiman JM, Hindley CE. Strategies to optimize treatment with NSAIDs in patients at risk for gastrointestinal and cardiovascular adverse events. Clin Ther. 2010;32(4):667–77.
Paul G, Udaya T, Steven W. A narrative review of the cardiovascular risks associated with concomitant aspirin and NSAID use. J Thromb Thrombolysis. 2019;47(1):16–30.
Iman S, Nafadi M, Faten F. Formulation of a fast-dissolving ketoprofen tablet using using freeze drying in blister technique. Drug Dev Ind Pharm. 2006;32:437–42.
Ebtessam A, Amira O, Ahmed M, Gamal M. Controlled precipitation for enhanced dissolution rate of flurbiprofen: development of rapidly disintegrating tablets. Drug Dev Ind Pharm. 2017;43(9):1430–9.
Adamo F, Valentina B, Gian CC, Celestino R, Carlos AFM. Fast dispersible/slow releasing ibuprofen tablets. Eur J Pharm Biopharm. 2008;69(1):335–41.
Khan KA. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27:48–9.
Zhang Y, Zhong D, Si D, Guo Y, Chen X, Zhou H. Lornoxicam pharmacokinetics in relation to cytochrome P 450 2C9 genotype. Br J Clin Pharmacol. 2005;59(1):14–7.
Chandrasekhar R, Hassan Z, Alhusban F, Smith AM, Mohammed AR. The role of formulation excipients in the development of lyophilised fast-disintegrating tablets. Eur J Pharm Biopharm. 2009;72:119–29.
AlHusban F, Perrie Y, Mohammed AR. Formulation and characterisation of lyophilised rapid disintegrating tablets using amino acids as matrix forming agents. Eur J Pharm Biopharm. 2010;75:254–62.
Shoukri RA, Ahmed IS, Shamma RN. In vitro and in vivo evaluation of nimesulide lyophilized orally disintegrating tablets. Eur J Pharm Biopharm. 2009;73:162–71.
Mizumoto T, Masuda Y, Yamamoto T, Yonemochi E, Terada K. Formulation design of a novel fast-disintegrating tablet. Int J Pharm. 2005;306:83–90.
Funding
This research was self-financed by the authors.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Faculty of Pharmacy, Cairo University, Egypt; ethical code PI:1721) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Nabarawi, M.A., Elshafeey, A.H., Mahmoud, D.M. et al. Fabrication, optimization, and in vitro/in vivo evaluation of diclofenac epolamine flash tablet. Drug Deliv. and Transl. Res. 10, 1314–1326 (2020). https://doi.org/10.1007/s13346-020-00709-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00709-4