Abstract
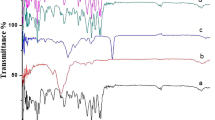
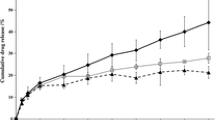
The present study aimed to develop matrix-type transdermal drug delivery system (TDDS) of metoprolol tartrate using polyvinyl pyrrolidone (PVP) and polyvinyl alcohol (PVA). The transdermal films were evaluated for physical parameters, Fourier transform infrared spectroscopy analysis (FTIR), differential scanning calorimetry (DSC), in vitro drug release, in vitro skin permeability, skin irritation test and stability studies. The films were found to be tough, non-sticky, easily moldable and possess good tensile strength. As the concentration of PVA was increased, the tensile strength of the films was also increased. Results of FTIR spectroscopy and DSC revealed the absence of any drug-polymer interactions. In vitro release of metoprolol followed zero-order kinetics and the mechanism of release was found to be diffusion rate controlled. In vitro release studies of metoprolol using Keshary-Chein (vertical diffusion cell) indicated 65.5 % drug was released in 24 h. In vitro skin permeation of metoprolol transdermal films showed 58.13 % of the drug was released after 24 h. In vitro skin permeation of metoprolol followed zero-order kinetics in selected formulations. The mechanism of release was found to be diffusion rate controlled. In a 22-day skin irritation test, tested formulation of transdermal films did not exhibit any allergic reactions, inflammation, or contact dermatitis. The transdermal films showed good stability in the 180-day stability study. It can be concluded that the TDDS of MPT can help in bypassing the first-pass effect and will provide patient improved compliance, without sacrificing the therapeutic advantages of the drugs.





Similar content being viewed by others
References
Sareen R, Jain N, Kumar D. An insight to osmotic drug delivery. Curr Drug Deliv. 2012;9(3):285–96.
Awasthi R, Kulkarni GT. Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: where do we stand? Drug Deliv. 2016;23(2):378–94.
Jain NK. Advances in controlled and novel drug delivery. 1st ed: CBS Publishers and Distributors; 2001. p. 426–36.
Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010;1(1):109–31.
Ahad A, Al-Jenoobi FI, Al-Mohizea AM, Akhtar N, Raish M, Aqil M. Systemic delivery of β-blockers via transdermal route for hypertension. Saudi Pharm J. 2015;23(6):587–602.
Nguyen KT, Ita KB, Parikh SJ, Popova IE, Bair DA. Transdermal delivery of captopril and metoprolol tartrate with microneedles. Drug Delivery Letters. 2014;4(3):236–43.
Bhatt DC, Dhake AS, Khar RK, Mishra DN. Development and in-vitro evaluation of transdermal matrix films of metoprolol tartrate. Yakugaku Zasshi. 2008;128(9):1325–31.
Aqil M, Sultana Y, Ali A, Dubey K, Najmi AK, Pillai KK. Transdermal drug delivery systems of a beta blocker: design, in vitro, and in vivo characterization. Drug Deliv. 2004;11(1):27–31.
Aqil M, Ali A, Sultana Y, Saha N. Comparative bioavailability of metoprolol tartrate after oral and transdermal administration in healthy male volunteers. Clinical Drug Investigation. 2007;27(12):833–9.
Aqil M, Sultana Y, Ali A. Matrix type transdermal drug delivery systems of metoprolol tartrate: In vitro characterization. Acta Pharma. 2003;53:119–25.
Sankar V, Johnson DB, Sivanand V, Ravichandran V, Raghuram S. Design and evaluation of nifedipine transdermal films. Indian J Pharm Sci. 2003;65:510–5.
Shinde AJ, Shinde AL, More HN. Design and evaluation of transdermal drug delivery system of gliclazide. Asian J Pharm. 2010;4:121–9.
Manvi FV, Dandagi PM, Gadad AP, Mastiholimath VS, Jagadesh T. Formulation of a transdermal drug delivery system of ketotifen fumarate. Indian J Pharm Sci. 2003;65:239–43.
Lec ST, Yac SH, Kim SW, Berner B. One way membrane for transdermal drug delivery system. II. System optimization. Int J Pharm. 1991;77(2–3):231–7.
Lewis S, Pandey S, Udupa N. Design and evaluation of matrix type and membrane controlled transdermal delivery systems of nicotine suitable for use in smoking cessation. Indian J Pharm Sci. 2006;68:179–84.
Satheeshababu BK, Shivakumar KL. Synthesis of conjugated chitosan and its effect on drug permeation from transdermal films. Indian J Pharm Sci. 2013;75(2):162–70.
Dua K, Pabreja K, Ramana MV. Aceclofenac topical dosage forms: in vitro and in vivo characterization. Acta Pharma. 2010;60:467–78.
Gungor S, Bektas A, Alp FI, Uydes-Dogan BS, Ozdemir O, Araman A, et al. Matrix-type transdermal films of verapamil hydrochloride: in vitro permeation studies through excised rat skin and pharmacodynamic evaluation in rats. Pharm Dev Technol. 2008;13(4):283–9.
Dua K, Chakravarthi S, Kumar D, Sheshala R, Gupta G. Formulation, characterization, in vitro, in vivo, and histopathological evaluation of transdermal drug delivery containing norfloxacin and Curcuma longa. International Journal of Pharmaceutical Investigation. 2013;3:183–7.
Dua K, Ramana MV, Madan J, Gupta G, Chakravarthi S, Awasthi R, et al. Norfloxacin and metronidazole topical formulations for effective treatment of bacterial infections and burn wounds. Interventional Medicine and Applied Science. 2016;8(2):68–76.
Jibry N, Murdan S. In vivo investigation, in mice and in man, in to the irritation potential of novel amphiphilogels being studied as transdermal drug carriers. Eur J Pharm Biopharm. 2004;58:107–19.
Aqil M, Zafar S, Ali A, Ahmad S. Transdermal drug delivery of labetolol hydrochloride: system development, in vitro, ex vivo and in vivo characterization. Curr Drug Deliv. 2005;2:125–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that this article has no conflict of interest.
Declaration of animal studies
The animal experimental protocol was approved by the Institutional Animal Ethical Committee. The animals were handled as per the guidelines of the Committee for the Purpose of Control and Supervision on Experimental animals (CPCSEA), New Delhi, India.
Rights and permissions
About this article
Cite this article
Malipeddi, V.R., Awasthi, R., Ghisleni, D.D.M. et al. Preparation and characterization of metoprolol tartrate containing matrix type transdermal drug delivery system. Drug Deliv. and Transl. Res. 7, 66–76 (2017). https://doi.org/10.1007/s13346-016-0334-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-016-0334-7