Abstract
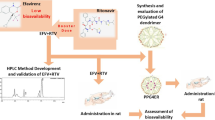
Local delivery of anti-HIV drugs to the colorectal mucosa, a major site of HIV replication, and their retention within mucosal tissue would allow for a reduction in dose administered, reduced dosing frequency and minimal systemic exposure. The current report describes a mucosal pre-exposure prophylaxis (mPrEP) strategy that utilizes nanocarrier conjugates (NC) consisting of poly(ethylene glycol) (PEG), amprenavir (APV), and a cell-penetrating peptide (CPP; namely Bac7, a fragment derived from bactenecin 7). APV-PEG NCs with linear PEGs (2, 5, 10, and 30 kDa) exhibited reduced (52–21 %) anti-HIV-1 protease (PR) activity as compared to free APV in an enzyme-based FRET assay. In MT-2 T cells, APV-PEG3.4 kDa-FITC (APF) anti-HIV-1 activity was significantly reduced (160-fold, IC50 = 8064 nM) due to poor cell uptake, whereas it was restored (IC50 = 78.29 nM) and similar to APV (IC50 = 50.29 nM) with the addition of Bac7 to the NC (i.e., APV-PEG3.4 kDa-Bac7, APB). Flow cytometry and confocal microscopy demonstrated Bac7-PEG3.4 kDa-FITC (BPF) uptake was two- and fourfold higher than APF in MT-2 T cells and Caco-2 intestinal epithelial cells, respectively. There was no detectable punctate fluorescence in either cell line suggesting that BPF directly enters the cytosol thus avoiding endosomal entrapment. After colorectal administration in mice, BPF mucosal concentrations were 21-fold higher than APF concentrations. BPF concentrations also remained constant for the 5 days of the study suggesting that (1) the NC’s structural characteristics (i.e., the size of the PEG carrier and the presence of a CPP) significantly influenced tissue persistence, and (2) the NCs were probably lodged in the lamina propria since the average rodent colon mucosal cell turnover time is 2–3 days. These encouraging results suggest that Bac7 functionalized NCs delivered locally to the colorectal mucosa may form drug delivery depots that are capable of sustaining colorectal drug concentrations. Although the exact mechanisms for tissue persistence are unclear and will require further study, these results provide proof-of-concept feasibility for mPrEP.














Similar content being viewed by others
References
Sheet WAF. Fact sheet N°360. WHO, http://www.who.int/mediacentre/factsheets/fs360/en/. 2015. http://www.who.int/mediacentre/factsheets/fs360/en/. Accessed 28 July 2015.
Mutua G, Sanders E, Mugo P, Anzala O, Haberer JE, Bangsberg D, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One. 2012;7(4):e33103.
Karim QA, Karim SSA, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi:10.1126/science.1193748.
Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–34. doi:10.1056/NEJMoa1110711.
Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. doi:10.1056/NEJMoa1108524.
Antoinette G, Nelson XZ, Ganapathi U, Szekely Z, Ganapathi U, Szekely Z, et al. Drug delivery strategies and systems for HIV/AIDS pre-exposure prophylaxis and treatment. J Control Release. 2015;219:669–80. doi:10.1016/j.jconrel.2015.08.042.
Nelson AG, Zhang X, Ganapathi U, Szekely Z, Flexner CW, Owen A, et al. Drug delivery strategies and systems for HIV/AIDS pre-exposure prophylaxis and treatment. J Control Release. 2015;219:669–80. doi:10.1016/j.jconrel.2015.08.042.
Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464(7286):217–23. doi:10.1038/nature08757.
Veazey RS, Lackner AA. Getting to the guts of HIV pathogenesis. J Exp Med. 2004;200(6):697–700. doi:10.1084/jem.20041464.
Yukl S, Wong JK. Blood and guts and HIV: preferential HIV persistence in GI mucosa. J Infect Dis. 2008;197(5):640–2. doi:10.1086/527325.
Karim SS, Kashuba AD, Werner L, Karim QA. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378(9787):279–81. doi:10.1016/S0140-6736(11)60878-7.
Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112):112re4. doi:10.1126/scitranslmed.3003174.
Schwartz JL, Rountree W, Kashuba AD, Brache V, Creinin MD, Poindexter A, et al. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1 % tenofovir gel. PLoS One. 2011;6(10):e25974. doi:10.1371/journal.pone.0025974.
Ferguson LM, Rohan LC. The importance of the vaginal delivery route for antiretrovirals in HIV prevention. Ther Deliv. 2011;2(12):1535–50. doi:10.4155/tde.11.126.
Hendrix CW, Chen BA, Guddera V, Hoesley C, Justman J, Nakabiito C, et al. MTN-001: randomized pharmacokinetic cross-over study comparing Tenofovir vaginal Gel and oral tablets in vaginal tissue and other compartments. PLoS ONE. 2013;8(1):e55013. doi:10.1371/journal.pone.0055013.
Hazra R, Balis FM, Tullio AN, DeCarlo E, Worrell CJ, Steinberg SM, et al. Single-dose and steady-state pharmacokinetics of Tenofovir disoproxil fumarate in human immunodeficiency virus-infected children. Antimicrob Agents Chemother. 2004;48(1):124–9. doi:10.1128/AAC.48.1.124-129.2004.
Kolate A, Baradia D, Patil S, Vhora I, Kore G, Misra A. PEG—a versatile conjugating ligand for drugs and drug delivery systems. J Control Release. 2014;192:67–81. doi:10.1016/j.jconrel.2014.06.046.
Allen A. Structure of gastrointestinal mucus glycoproteins and the viscous and gel-forming properties of mucus. Br Med Bull. 1978;34(1):28–33.
Norris DA, Puri N, Sinko PJ. The effect of physical barriers and properties on the oral absorption of particulates. Adv Drug Deliv Rev. 1998;34(2–3):135–54. doi:10.1016/S0169-409X(98)00037-4.
Norris DA, Sinko PJ. Effect of size, surface charge, and hydrophobicity on the translocation of polystyrene microspheres through gastrointestinal mucin. J Appl Polym Sci. 1997;63(11):1481–92.
Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv Drug Deliv Rev. 2012;64(6):557–70. doi:10.1016/j.addr.2011.12.009.
Willits RK, Saltzman WM. Synthetic polymers alter the structure of cervical mucus. Biomaterials. 2001;22(5):445–52. doi:10.1016/S0142-9612(00)00197-6.
Silberberg A, Meyer FA. Structure and function of mucus. Adv Exp Med Biol. 1982;144:53–74.
Silberberg A, Meyer FA. Structure and function of mucus. Mucus in health and disease II. New York: Plenum Press; 1982.
Ramsey JD, Flynn NH. Cell-penetrating peptides transport therapeutics into cells. Pharmacol Ther. 2015. doi:10.1016/j.pharmthera.2015.07.003.
Zhang X, Jin Y, Plummer MR, Pooyan S, Gunaseelan S, Sinko PJ. Endocytosis and membrane potential are required for HeLa cell uptake of R.I.-CKTat9, a retro-inverso tat cell penetrating peptide. Mol Pharm. 2009;6(3):836–48. doi:10.1021/mp800121f.
Brock R. The uptake of arginine-rich cell-penetrating peptides: putting the puzzle together. Bioconjug Chem. 2014;25(5):863–8. doi:10.1021/bc500017t.
Pujals S, Giralt E. Proline-rich, amphipathic cell-penetrating peptides. Adv Drug Deliv Rev. 2008;60(4–5):473–84. doi:10.1016/j.addr.2007.09.012.
Sadler K, Eom KD, Yang JL, Dimitrova Y, Tam JP. Translocating proline-rich peptides from the antimicrobial peptide bactenecin 7. Biochemistry. 2002;41(48):14150–7. doi:10.1021/bi026661l.
Eissenstat M, Guerassina T, Gulnik S, Afonina E, Silva AM, Ludtke D, et al. Enamino-oxindole HIV protease inhibitors. Bioorg Med Chem Lett. 2012;22(15):5078–83. doi:10.1016/j.bmcl.2012.05.120.
Ellman GL. A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys. 1958;74(2):443–50. D - CLML: 5834:27208:574 OTO - NLM.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63. doi:10.1016/0022-1759(83)90303-4.
Matayoshi ED, Wang GT, Krafft GA, Erickson J. Novel fluorogenic substrates for assaying retroviral proteases by resonance energy transfer. Science. 1990;247(4945):954–8.
Nara PL, Hatch WC, Dunlop NM, Robey WG, Arthur LO, Gonda MA, et al. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res Hum Retrovir. 1987;3(3):283–302.
Spreen WR, Margolis DA, Pottage JC. Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS. 2013;8(6):565–71. doi:10.1097/COH.0000000000000002.
Flexner C, Saag M. The antiretroviral drug pipeline: prospects and implications for future treatment research. Curr Opin HIV AIDS. 2013;8(6):572–8. doi:10.1097/COH.0000000000000011.
Dolgin E. Long-acting HIV, drugs advanced to overcome adherence challenge. Nat Med. 2014;20(4):323–4.
Friend DR, Kiser PF. Assessment of topical microbicides to prevent HIV-1 transmission: concepts, testing, lessons learned. Antivir Res. 2013;99(3):391–400. doi:10.1016/j.antiviral.2013.06.021.
Garg AB, Nuttall J, Romano J. The future of HIV microbicides: challenges and opportunities. Antivir Chem Chemother. 2009;19(4):143–50.
Smith JM, Srinivasan P, Teller RS, Lo Y, Dinh CT, Kiser PF, et al. Tenofovir disoproxil fumarate intravaginal ring protects high dose depot medroxyprogesterone acetate treated macaques from multiple SHIV exposures. J Acquir Immune Defic Syndr. 2014. doi:10.1097/QAI.0000000000000402.
Zhang W, Hu M, Shi Y, Gong T, Dezzutti CS, Moncla B, et al. Vaginal microbicide film combinations of two reverse transcriptase inhibitors, EFdA and CSIC, for the prevention of HIV-1 sexual transmission. Pharm Res. 2015;32(9):2960–72. doi:10.1007/s11095-015-1678-2.
Deeks ED. Darunavir: a review of its use in the management of HIV-1 infection. Drugs. 2014;74(1):99–125. doi:10.1007/s40265-013-0159-3.
Herold DA, Keil K, Bruns DE. Oxidation of polyethylene glycols by alcohol dehydrogenase. Biochem Pharmacol. 1989;38(1):73–6. doi:10.1016/0006-2952(89)90151-2.
Bendele A, Seely J, Richey C, Sennello G, Shopp G. Short communication: renal tubular vacuolation in animals treated with polyethylene-glycol-conjugated proteins. Toxicol Sci. 1998;42(2):152–7. doi:10.1006/toxs.1997.2396.
Cho WS, Cho M, Jeong J, Choi M, Cho HY, Han BS, et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol Appl Pharmacol. 2009;236(1):16–24. doi:10.1016/j.taap.2008.12.023.
Duncan R, Richardson SCW. Endocytosis and intracellular trafficking as gateways for nanomedicine delivery: opportunities and challenges. Mol Pharm. 2012;9(9):2380–402. doi:10.1021/mp300293n.
Johnson BM, Charman WN, Porter CJH. An in vitro examination of the impact of polyethylene glycol 400, Pluronic P85, and vitamin E d-α-tocopheryl polyethylene glycol 1000 succinate on P-glycoprotein efflux and enterocyte-based metabolism in excised rat intestine. AAPS PharmSci. 2002;4(4):E40.
Shen Q, Lin Y, Handa T, Doi M, Sugie M, Wakayama K, et al. Modulation of intestinal P-glycoprotein function by polyethylene glycols and their derivatives by in vitro transport and in situ absorption studies. Int J Pharm. 2006;313(1–2):49–56. doi:10.1016/j.ijpharm.2006.01.020.
Hugger ED, Audus KL, Borchardt RT. Effects of poly(ethylene glycol) on efflux transporter activity in Caco-2 cell monolayers. J Pharm Sci. 2002;91(9):1980–90. doi:10.1002/jps.10175.
Choi JS, Jo BW. Enhanced paclitaxel bioavailability after oral administration of pegylated paclitaxel prodrug for oral delivery in rats. Int J Pharm. 2004;280(1–2):221–7. doi:10.1016/j.ijpharm.2004.05.014.
Wang YY, Lai SK, Suk JS, Pace A, Cone R, Hanes J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew Chem Int Ed. 2008;47(50):9726–9. doi:10.1002/anie.200803526.
Olmsted SS, Padgett JL, Yudin AI, Whaley KJ, Moench TR, Cone RA. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81(4):1930–7.
Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;17(15–16):850–60. doi:10.1016/j.drudis.2012.03.002.
Ziegler AE, Seelig J. Contributions of glycosaminoglycan binding and clustering to the biological uptake of the nonamphipathic cell-penetrating peptide WR 9. Biochemistry. 2011;50(21):4650–64. doi:10.1021/bi1019429.
Salomone F, Cardarelli F, Di Luca M, Boccardi C, Nifosì R, Bardi G, et al. A novel chimeric cell-penetrating peptide with membrane-disruptive properties for efficient endosomal escape. J Control Release. 2012;163(3):293–303. doi:10.1016/j.jconrel.2012.09.019.
El-Sayed A, Futaki S, Harashima H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: ways to overcome endosomal entrapment. AAPS J. 2009;11(1):13–22. doi:10.1208/s12248-008-9071-2.
Lipkin M. Proliferation and differentiation of normal and diseased gastrointestinal cells. Physiology of the gastrointestinal tract. New York: Raven; 1987.
Zhang W. Inventor Preparation of L-nucleoside derivatives as antiviral agents. China patent CN 1465574, 2004.
Mingyan Zhao SZ. Inventor preparation of acyclic nucleotides as antiviral agents. China patent CN 1966514 A 20070523, 2007.
Acknowledgments
Financial support from NIH MERIT AI51214, AI117776, and the Parke-Davis Endowed Chair in Pharmaceutics and Drug Delivery is gratefully acknowledged. Flow cytometry/Cell sorting CORE facility at Rutgers, The State University of New Jersey is acknowledged for performing flow cytometry and confocal microscopy. The human CD4+ MT-2 T cells were obtained from Dr. Douglas Richman, courtesy the NIH AIDS research and reference program, division of AIDS, NIAID (NIH cat # 237). A fellowship from the American Foundation for Pharmaceutical Education to M. Palombo is also acknowledged.
All institutional and national guidelines for the care and use of laboratory animals were followed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samizadeh, M., Zhang, X., Gunaseelan, S. et al. Colorectal delivery and retention of PEG-Amprenavir-Bac7 nanoconjugates—proof of concept for HIV mucosal pre-exposure prophylaxis. Drug Deliv. and Transl. Res. 6, 1–16 (2016). https://doi.org/10.1007/s13346-015-0269-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-015-0269-4