Abstract
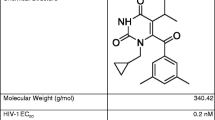
UC781 is a potent nonnucleoside reverse transcriptase inhibitor being investigated as a potential microbicide to prevent transmission of HIV-1 both vaginally and rectally. This study was designed to investigate the in vitro drug release, in vitro permeability/safety, and in vivo pharmacokinetics in rabbits of a vaginal gel prepared with micronized or nonmicronized UC781 (UC781m and UC781nm, respectively). Gels prepared with UC781m had greater in vitro release rates (Franz cells) and permeability/tissue-associated UC781 concentrations than gels prepared with UC781nm (EpiVaginal tissues). Both gels were well tolerated under in vitro conditions compared with controls using EpiVaginal tissues. Following intravaginal administration of both gels to rabbits, tissue concentrations typically ranged from 1,000 to over 10,000 ng/g regardless of dosing regimen (single dose or 7 days once daily dosing) and sampling times (2 and 24 h post-dose). Tissue-associated concentrations were highly variable, and no statistically significant differences were found between test conditions. Plasma levels were generally low after vaginal administration: following a single dose, the concentrations were between 0.5 and 1.0 ng/mL. After 7 days repeated once daily dosing, UC781 concentrations were slightly higher ranging from below 1.0 to about 2 ng/mL, although none of the differences were statistically significant. Based on these results, gels prepared with either form of UC781 led to tissue concentrations well in excess of UC781’s EC50 under in vitro conditions (~3 ng/mL).




Similar content being viewed by others
References
Balzarini J, Brouwer WG, Dao DC, Osika EM, de Clercq E. Identification of novel thiocarboxanilide derivatives that suppress a variety of drug-resistant mutant human immunodeficiency virus type 1 strains at a potency similar to that for wild-type virus. Antimicrob Agents Chemother. 1996;40:1454–66.
Balzarini J, Naesens L, Verbeken E, LAGA M, van Damme L, Parniak M, et al. Preclinical studies on thiocarboxanilide UC-781 as a virucidal agent. AIDS. 1998;12:1129–38.
Borkow G, Arion D, Wainberg MA, Parniak MA. The thiocarboxanilide nonnucleoside inhibitor UC781 restores antiviral activity of 3′-azido-3′-deoxythymidine (AZT) against AZT-resistant human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1999;43:259–63.
Pelemans H, Esnouf R, DE Clercq E, Balzarini J. Mutational analysis of trp-229 of human immunodeficiency virus type 1 reverse transcriptase (RT) identifies this amino acid residue as a prime target for the rational design of new non-nucleoside RT inhibitors. Mol Pharmacol. 2000;57:954–60.
van Herrewege Y, Michiels J, van Roey J, Fransen K, Kestens L, Balzarini J, et al. In vitro evaluation of nonnucleoside reverse transcriptase inhibitors UC-781 and TMC120-R147681 as human immunodeficiency virus microbicides. Antimicrob Agents Chemother. 2004;48:337–9.
Fletcher P, Kiselyeva Y, Wallace G, Romano J, Griffin G, Margolis L, et al. The nonnucleoside reverse transcriptase inhibitor UC-781 inhibits human immunodeficiency virus type 1 infection of human cervical tissue and dissemination by migratory cells. J Virol. 2005;79:11179–86.
Zussman A, Lara L, Lara HH, Bentwich Z, Borkow G. Blocking of cell-free and cell-associated HIV-1 transmission through human cervix organ culture with UC781. AIDS. 2003;17:653–61.
Borkow G, Barnard J, Nguyen TM, Belmonte A, Wainberg MA, Parniak MA. Chemical barriers to human immunodeficiency virus type 1 (HIV-1) infection: retrovirucidal activity of UC781, a thiocarboxanilide nonnucleoside inhibitor of HIV-1 reverse transcriptase. J Virol. 1997;71:3023–30.
Schwartz JL, Kovalevsky G, Lai JJ, Ballagh SA, Mccormick T, Douville K, et al. A randomized six-day safety study of an antiretroviral microbicide candidate UC781, a non-nucleoside reverse transcriptase inhibitor. Sex Transm Dis. 2008;35:414–9.
Thakker KD, Chern WC. Development and validation of in vitro release tests for semisolid dosage forms-Case study. Dissolution Technologies, May, 2003;10–16.
Tien D, Schnaare RL, Kang F, Cohl G, Mccormick TJ, Moench TR, et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21:845–53.
Ayehunie S, Cannon C, Lamore S, Kubilus J, Anderson DJ, Pudney J, et al. Organotypic human vaginal-ectocervical tissue model for irritation studies of spermicides, microbicides, and feminine-care products. Toxicol In Vitro. 2006;20:689–98.
Ayehunie S, Cannon C, Larosa K, Pudney J, Anderson DJ, Klausner M. Development of an in vitro alternative assay method for vaginal irritation. Toxicology. 2011;279:130–8.
Fletcher PS, Harman SJ, Boothe AR, Doncel GF, Shattock RJ. Preclinical evaluation of lime juice as a topical microbicide candidate. Retrovirology. 2008;5:3.
Nuttall JP, Thake DC, LEWIS MG, Ferkany JW, Romano JW, Mitchnick MA. Concentrations of dapivirine in the rhesus macaque and rabbit following once daily intravaginal administration of a gel formulation of [14C]dapivirine for 7 days. Antimicrob Agents Chemother. 2008;52:909–14.
Acknowledgments
The support of Missy Peet and Devon Kyle of MPI in coordination of the rabbit studies and sample bioanalytical analysis is gratefully acknowledged. The contributions of Seyoum Ayehunie of MatTek, Inc. and Qing Wang at Absorption Systems LP. are also acknowledged. The views of the authors do not necessarily reflect those of USAID.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was funded by the United States Agency for International Development (USAID) under Cooperative Agreement GPO-A-00-0005-00.
Rights and permissions
About this article
Cite this article
Clark, M.R., McCormick, T.J., Doncel, G.F. et al. Preclinical evaluation of UC781 microbicide vaginal drug delivery. Drug Deliv. and Transl. Res. 1, 175–182 (2011). https://doi.org/10.1007/s13346-011-0019-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-011-0019-1