Abstract
Introduction
The peri-operative use of high-dose dexamethasone to reduce cerebral oedema may result in worsening glycaemic control in people with diabetes and glucocorticoid-induced diabetes in susceptible individuals. This study aims to examine the incidence of glucocorticoid-induced diabetes in a cohort of neurosurgical patients receiving high-dose dexamethasone peri-operatively.
Materials and methods
Adult non-diabetic neurosurgical patients receiving high-dose dexamethasone were prospectively studied. Exclusion criteria included pregnancy, HbA1c > 6.0%, and use of anti-diabetes therapies. The following data were collected: Family history of diabetes, body mass index, fasting glucose, insulin, C-peptide, and HbA1c (prior to surgery and 6 weeks after last dose of dexamethasone). Homeostatic model assessment values were calculated. Peri-operative glucose readings were recorded and 75 g oral glucose tolerance tests performed at the end of 6 weeks. Paired student t tests and multiple linear regressions were used.
Results
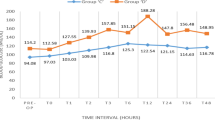
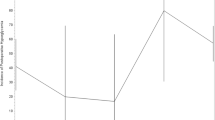
Data from 21 participants (11 women) were available. The mean total dose of dexamethasone was 96 ± 34 mg, and treatment duration was 17 ± 7 days. A total of 105 random blood glucose levels were documented peri-operatively (mean 7.0 ± 1.0 mmol/L). Six weeks following cessation of dexamethasone course, none of the participants developed diabetes, defined either by fasting glucose or by 75 g OGTT. There was a statistically significant increase in the mean HOMA-β from 81.5 to 102.1% (p = 0.01) and a significant decrease in the mean fasting glucose from 5.7 to 4.8 mmol/L (p = 0.001).
Conclusions
The use of high-dose dexamethasone in this cohort of neurosurgical patients did not result in glucocorticoid-induced diabetes. Hyperglycaemia was transient and had resolved by 6 weeks.


Similar content being viewed by others
References
Abdelmannan D, Tahboub R, Genuth S, Ismail-Beigi F. Effect of dexamethasone on oral glucose tolerance in healthy adults. Endocr Pract. 2010;16(5):770–7.
Kaal EC, Vecht CJ. The management of brain edema in brain tumors. Curr Opin Oncol. 2004;16(6):593–600.
Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol. 2003;43(11):1216–27.
Abdelmalak BB, Bonilla AM, Yang D, Chowdary HT, Gottlieb A, Lyden SP, et al. The hyperglycemic response to major noncardiac surgery and the added effect of steroid administration in patients with and without diabetes. Anesth Analg. 2013;116(5):1116–22.
Lukins MB, Manninen PH. Hyperglycemia in patients administered dexamethasone for craniotomy. Anesth Analg. 2005;100(4):1129–33.
Hans P, Vanthuyne A, Dewandre P-Y, Brichant J-F, Bonhomme V. Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetic patients undergoing abdominal surgery. Br J Anaesth. 2006;97(2):164–70.
Sethi R, Naqash IA, Bajwa SJS, Dutta V, Ramzan AU, Zahoor SA. Evaluation of hyperglycaemic response to intra-operative dexamethasone administration in patients undergoing elective intracranial surgery: a randomised, prospective study. Asian J Neurosurg. 2016;11(2):98–102.
Abroug F, Ouanes-Besbes L, Fkih-Hassen M, Ouanes I, Ayed S, Dachraoui F, et al. Prednisone in COPD exacerbation requiring ventilatory support: an open-label randomised evaluation. Eur Respir J. 2014;43(3):717–24.
Alexander TH, Weisman MH, Derebery JM, Espeland MA, Gantz BJ, Gulya AJ, et al. Safety of high-dose corticosteroids for the treatment of autoimmune inner ear disease. Otol Neurotol. 2009;30(4):443–8.
Fong AC, Cheung NW. The high incidence of steroid-induced hyperglycaemia in hospital. Diabetes Res Clin Pract. 2013;99(3):277–80.
Boloori A, Saghafian S, Chakkera HA, Cook CB. Characterization of remitting and relapsing hyperglycemia in post-renal-transplant recipients. PLoS ONE. 2015;10(11):e0142363.
Gonzalez-Gonzalez JG, Mireles-Zavala LG, Rodriguez-Gutierrez R, Gomez-Almaguer D, Lavalle-Gonzalez FJ, Tamez-Perez HE, et al. Hyperglycemia related to high-dose glucocorticoid use in noncritically ill patients. Diabetol Metab Syndr. 2013;5(1):18.
Pilkey J, Streeter L, Beel A, Hiebert T, Li X. Corticosteroid-induced diabetes in palliative care. J Palliat Med. 2012;15(6):681–9.
Yoo K-E, Kang RY, Lee J-Y, Lee YJ, Suh SY, Kim KS, et al. Awareness of the adverse effects associated with prophylactic corticosteroid use during docetaxel therapy. Support Care Cancer. 2015;23(7):1969–77.
Rowbottom L, Stinson J, McDonald R, Emmenegger U, Cheng S, Lowe J, et al. Retrospective review of the incidence of monitoring blood glucose levels in patients receiving corticosteroids with systemic anti-cancer therapy. Ann Palliat Med. 2015;4(2):70–7.
Jeong Y, Han HS, Lee HD, Yang J, Jeong J, Choi MK, et al. A Pilot Study evaluating steroid-induced diabetes after antiemetic dexamethasone therapy in chemotherapy-treated cancer patients. Cancer Res Treat. 2016;48(4):1429.
X-X Liu, X-M Zhu, Miao Q, Ye H-Y, Zhang Z-Y, Li Y-M. Hyperglycemia induced by glucocorticoids in nondiabetic patients: a meta-analysis. Ann Nutr Metab. 2014;65(4):324–32.
Sonabend RY, McKay SV, Okcu MF, Yan J, Haymond MW, Margolin JF. Hyperglycemia during induction therapy is associated with poorer survival in children with acute lymphocytic leukemia. J Pediatr. 2009;155(1):73–8.
Weiser MA, Cabanillas ME, Konopleva M, Thomas DA, Pierce SA, Escalante CP, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate–cytarabine regimen. Cancer. 2004;100(6):1179–85.
Organization WH. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Geneva: World Hearth Organisation; 2006.
World Health Organization. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus: abbreviated report of a WHO consultation 2011. Geneva: World Health Organization; 2013.
University of Oxford OCfD, endocrinology and metabolism, diabetes trials unit. HOMA calculator. Oxford (England): https://www.dtu.ox.ac.uk/homacalculator/download.php.
Nazar CE, Lacassie HJ, Lopez RA, Munoz HR. Dexamethasone for postoperative nausea and vomiting prophylaxis: effect on glycaemia in obese patients with impaired glucose tolerance. Eur J Anaesthesiol. 2009;26(4):318–21.
Fizazi K, Chi KN, de Bono JS, Gomella LG, Miller K, Rathkopf DE, et al. Low incidence of corticosteroid-associated adverse events on long-term exposure to low-dose prednisone given with abiraterone acetate to patients with metastatic castration-resistant prostate cancer. Eur Urol. 2016;70(3):438–44.
Iwamoto T, Kagawa Y, Naito Y, Kuzuhara S, Kojima M. Steroid induced diabetes mellitus and related risk factors in patients with neurologic diseases. Pharmacotherapy. 2004;24(4):5–514.
den Uyl D, van Raalte DH, Nurmohamed MT, Lems WF, Bijlsma JW, Hoes JN, et al. Metabolic effects of high-dose prednisolone treatment in early rheumatoid arthritis: balance between diabetogenic effects and inflammation reduction. Arthritis Rheumatol. 2012;64(3):639–46.
Pirsch J, Henning A, First M, Fitzsimmons W, Gaber AO, Reisfield R, et al. New-onset diabetes after transplantation: results from a double-blind early corticosteroid withdrawal trial. Am J Transplant. 2015;15(7):1982–90.
Burt MG, Willenberg VM, Petersons CJ, Smith MD, Ahern MJ, Stranks SN. Screening for diabetes in patients with inflammatory rheumatological disease administered long-term prednisolone: a cross-sectional study. Rheumatology. 2012;51(6):1112–9.
Kumar PR, Bhansali A, Ravikiran M, Bhansali S, Dutta P, Thakur J, et al. Utility of glycated hemoglobin in diagnosing type 2 diabetes mellitus: a community-based study. J Clin Endocrinol Metab. 2010;95(6):2832–5.
Hoes J, Van Der Goes M, Van Raalte D, Van Der Zijl N, Den Uyl D, Lems W, et al. Glucose tolerance, insulin sensitivity and β-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70(11):1887–94.
Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Assefa Z, Akbib S, Lavens A, Stangé G, Ling Z, Hellemans KH, et al. Direct effect of glucocorticoids on glucose-activated adult rat β-cells increases their cell number and their functional mass for transplantation. Am J Physiol-Endocrinol Metab. 2016;311(4):E698–705.
Choi SB, Jang JS, Hong SM, Jun DW, Park S. Exercise and dexamethasone oppositely modulate β-cell function and survival via independent pathways in 90% pancreatectomized rats. J Endocrinol. 2006;190(2):471–82.
Ogawa A, Johnson JH, Ohneda M, McAllister CT, Inman L, Alam T, et al. Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes. J Clin Invest. 1992;90(2):497.
Acknowledgements
The authors would like to thank the nursing staff at Ward 2 and the Neurosurgeons working at Macquarie University Hospital for their help in recruiting the patients. Also, many thanks to Douglas-Hanley Moir Pathology for their help in performing the blood tests.
Funding
This research did not receive any specific grant from any funding agency.
Author information
Authors and Affiliations
Contributions
MA recruited the participants, performed the statistical analysis and drafted the manuscript. KH designed the study, reviewed and edited the manuscript. ML reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
About this article
Cite this article
Alabbood, M., Ling, M. & Ho, K. Effect of high-dose dexamethasone on patients without diabetes during elective neurosurgery: a prospective study. Diabetol Int 10, 109–116 (2019). https://doi.org/10.1007/s13340-018-0370-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-018-0370-2