Abstract
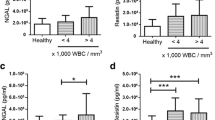
The role of oxidative stress in the pathogenesis of dengue infection is not completely known. A recent study reveals the involvement of oxidative stress responsive molecules in the generation of host immune responses to dengue virus in vitro. Objective of the present study was to analyse the changes in the expression of oxidant-antioxidant genes Nox-2 (NADPH oxidase) and Nrf2 (nuclear factor-erythroid 2-related factor 2) in patients with dengue during the early phase of infection compared to other febrile illness (OFI) cases and healthy controls using Real-time qPCR assay. The study enrolled 88 dengue patients, 31 OFI cases, and 63 healthy individuals as controls. Out of 88 dengue cases, 32 were classified as severe dengue cases (SD) and remaining 56 patients as non-severe dengue (NSD). Blood samples were collected firstly at the time of admission and a second sampling was done from the available individuals (38 dengue and 13 OFI cases) at the time of defervescence. Total RNA was extracted from the Peripheral blood mononuclear cells and the transcripts level of Nox-2 and Nrf2 were analysed by qPCR. On DOA, both Nox-2 and Nrf2 expression was found to be down regulated in dengue and OFI cases (P < 0.05) compared to healthy controls. Interestingly at defervescence, the transcript levels were found to be significantly increased in dengue cases unlike OFI, where no such increment was evidenced. From DOA to DOD, the study observed a signficant increase in the levels of Nox-2 transcripts (P < 0.05) both in SD and NSD cases. But a significant Nrf2 activation was not observed in SD cases as we found in NSD cases. Thus a steady and significant increase in Nox-2 transcript level in severe, non-severe and secondary dengue infected groups observed in the current study supports the earlier reports on the involvement of anti-oxidant response in dengue severity. However further studies on its protein levels and mechanistic action would decipher the exact role of these potential molecules in the disease virulence.


Similar content being viewed by others
References
Beatty ME, Stone A, Fitzsimons DW, Hanna JN, Lam SK, Vong S, et al. Best practices in dengue surveillance: a report from the Asia-Pacific and Americas Dengue Prevention Boards. PLoS Negl Trop Dis. 2010;4(11):e890.
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19.
Calzavara-Silva CE, Gomes ALV, Maia RCC, Acioli-Santos B, Gil LHVG, Marques Jr ETA. Early molecular markers predictive of dengue hemorrhagic fever. An Acad Bras Ciênc. 2009;81(4):671–7.
Carvajal-Yepes M, Himmelsbach K, Schaedler S, Ploen D, Krause J, Ludwig L, et al. Hepatitis C virus impairs the induction of cytoprotective Nrf2 target genes by delocalization of small Maf proteins. J Biol Chem. 2011;286(11):8941–51.
Green S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A, et al. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J Infect Dis. 1999;179(4):755–62.
Halstead SB, Lum LC. Assessing the prognosis of dengue-infected patients. F1000 Med Rep. 2009;1.
Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36(10):1199–207.
Jung T, Bader N, Grune T. Oxidized proteins: intracellular distribution and recognition by the proteasome. Arch Biochem Biophys. 2007;462(2):231–7.
Khurram M, Qayyum W, Hassan SJU, Mumtaz S, Bushra HT, Umar M. Dengue hemorrhagic fever: comparison of patients with primary and secondary infections. J Infect Public Health. 2014;7(6):489–95.
Klassen P, Biesalski HK, Mazariegos M, Solomons NW, Fürst P. Classic dengue fever affects levels of circulating antioxidants. Nutr Burbank Los Angel Cty Calif. 2004;20(6):542–7.
Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4(3):181–9.
Martina BEE, Koraka P, Osterhaus ADME. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22(4):564–81.
Myers RM, Varkey MJ, Reuben R, Jesudass ES. Dengue outbreak in Vellore, southern India, in 1968, with isolation of four dengue types from man and mosquitoes. Indian J Med Res. 1970;58(1):24–30.
Olagnier D, Peri S, Steel C, van Montfoort N, Chiang C, Beljanski V, et al. Cellular oxidative stress response controls the antiviral and apoptotic programs in dengue virus-infected dendritic cells. PLoS Pathog. 2014;10(12):e1004566.
Oyadomari S, Yun C, Fisher EA, Kreglinger N, Kreibich G, Oyadomari M, et al. Cotranslocational degradation protects the stressed endoplasmic reticulum from protein overload. Cell. 2006;126(4):727–39.
Rajendiran S, Lakshamanappa HS, Zachariah B, Nambiar S. Desialylation of plasma proteins in severe dengue infection: possible role of oxidative stress. Am J Trop Med Hyg. 2008;79(3):372–7.
Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res Rev Bras Pesqui Médicas E Biológicas Soc Bras Biofísica Al. 2005;38(7):995–1014.
Shepard DS, Halasa YA, Tyagi BK, Adhish SV, Nandan D, Karthiga KS, et al. Economic and disease burden of dengue illness in India. Am J Trop Med Hyg. 2014;91(6):1235–42.
Singhi S, Kissoon N, Bansal A. Dengue and dengue hemorrhagic fever: management issues in an intensive care unit. J Pediatr (Rio J). 2007;83(2 Suppl):S22–35.
Soucy-Faulkner A, Mukawera E, Fink K, Martel A, Jouan L, Nzengue Y, et al. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 2010;6(6):e1000930.
Soundravally R, Hoti SL, Patil SA, Cleetus CC, Zachariah B, Kadhiravan T, et al. Association between proinflammatory cytokines and lipid peroxidation in patients with severe dengue disease around defervescence. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2014;18:68–72.
Soundravally R, Sankar P, Bobby Z, Hoti SL. Oxidative stress in severe dengue viral infection: association of thrombocytopenia with lipid peroxidation. Platelets. 2008;19(6):447–54.
Soundravally R, Sankar P, Hoti SL, Selvaraj N, Bobby Z, Sridhar MG. Oxidative stress induced changes in plasma protein can be a predictor of imminent severe dengue infection. Acta Trop. 2008;106(3):156–61.
Soundravally R, Sherin J, Agieshkumar BP, Daisy MS, Cleetus C, Narayanan P, et al. Serum levels of copper and iron in dengue fever. Rev Inst Med Trop São Paulo. 2015;57(4):315–20.
Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci CMLS. 2002;59(9):1428–59.
Yageta Y, Ishii Y, Morishima Y, Masuko H, Ano S, Yamadori T, et al. Role of Nrf2 in host defense against influenza virus in cigarette smoke-exposed mice. J Virol. 2011;85(10):4679–90.
Acknowledgements
This work was supported by Indian council of medical research (ICMR) (Ref no: VIR/RCI/AES/58/2014/ECD-I) and intramural research grant from Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER) (Ref no:JIP/Res/Intra-PhD/01/2014).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cherupanakkal, C., Ramachadrappa, V., Kadhiravan, T. et al. Differential expression of NADPH oxidase-2 (Nox-2) and nuclear factor-erythroid 2-related factor 2 (Nrf2) transcripts in peripheral blood mononuclear cells isolated from dengue patients. VirusDis. 28, 54–60 (2017). https://doi.org/10.1007/s13337-017-0365-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-017-0365-9