Abstract
Backgroud
The interactions between Chinese herbs and drugs pose a great challenge to the combined clinical application of Chinese herbs and drugs. Chinese medicinal products contain complex pharmacologically active components that may influence the in vivo processes of drugs in a variety of ways. In China, drugs based on Panax ginseng total saponins (PNS) are often combined with warfarin for the treatment of cardiovascular diseases.
Objectives
To assess the effects of Panax notoginseng saponins (PNS) on the pharmacokinetics of warfarin and its mechanism.
Method
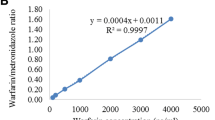
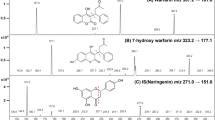
Blood was collected for the determination of the prothrombin time (PT) and international normalized ratio (INR) from rats treated with warfarin alone or with warfarin + PNS. The plasma concentration of warfarin was determined by high-performance liquid chromatography. Western blot was used to detect the expression of cytochrome P450 (CYP) enzymes.
Results
When warfarin and PNS were co-administered, the PT and INR increased compared to when warfarin was given alone. 72 hours after administration, compared to the warfarin alone group, the warfarin + low-dose PNS, warfarin + medium-dose PNS, and warfarin + high-dose PNS groups showed 110%, 122%, and 126% increases in PT, respectively (all P < 0.05), as well as 111%, 124%, and 128% increases in INR (all P < 0.05). Compared with the warfarin alone group, the clearance rate (CL/F) of warfarin in the warfarin + low-dose PNS, warfarin + medium-dose PNS, and warfarin + high-dose PNS groups was 10% (P > 0.05), 23% (P < 0.05), and 33% (P < 0.05) lower, respectively, while the systemic exposure (area under the concentration-time curve, AUC0–t) increased by 106% (P > 0.05), 119% (P < 0.05), and 134% (P < 0.05), respectively, and the blood concentration of warfarin incresed by 112%, 113%, and 114%, respectively (all P > 0.05). After combined treatment of HepG2 cells with warfarin + PNS, CYP1A2 expression was upregulated (P < 0.05) and CYP3A4 was downregulated (P < 0.05) but there was no effect on CYP2C9. In animal experiments, PNS had different effect on the expression of CYP1A2 in different doses. While a low dose of PNS resulted in downregulated CYP1A2 expression (P < 0.05), a medium dose resulted in upregulation (P < 0.05), and CYP1A2 expression was not significantly affected by a high dose of PNS (P > 0.05). Meanwhile, PNS at all doses downregulated the expression of CYP3A4 (P < 0.05) but had no effect on the expression of CYP2C9 (P > 0.05).
Conclusion
PNS can increase the blood concentration of warfarin, as well as the exposure time, and it can enhance the anticoagulant effect of warfarin by inhibiting the expression of the liver enzyme CYP3A4.







Similar content being viewed by others
Change history
22 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s13318-022-00761-0
References
Wen MS, Lee MT. Warfarin pharmacogenetics: new life for an old drug. Acta Cardiol Sin. 2013;29:235–42. https://doi.org/10.5935/abc.20130106.
Kearon C, Akl EA, Ornelas J, Blaivas A, et al. Antithrombotic therapy for VTE disease: chest guideline and expert panel report. Chest. 2016;149:315–52. https://doi.org/10.1016/j.chest.2015.11.026.
Zhang J-H, Liu M-B, Guan C-F, et al. Establishment and practice of online anticoagulation clinic. Chin J Pharm. 2014;16:1476–8. https://doi.org/10.11669/cpj.2014.16.024.
Wang Y, Tan X-P, Yan P-K. A case of abnormal INR caused by warfarin in patients with atrial fibrillation complicated with coronary heart disease. China Pharmacist. 2015;18(1):105–8.
Zhang J, Chen Z, Chen C. Impact of CYP2C9, VKORC1 and CYP4F2 genetic polymorphisms on maintenance warfarin dosage in Han-Chinese patients: a systematic review and meta-analysis. Meta Gene. 2016;9:197–209. https://doi.org/10.1016/j.mgene.2016.07.002.
Hirsh JV, Fuster V, Halperin JL. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. J Am Coll Cardiol. 2003;4l(9):1633–52. https://doi.org/10.1161/01.CIR.0000063575.17904.4E.
Breckenridge A, Orme M. Kinetics of warfarin absorption in man. Clin Pharmacol Ther. 1973;14(6):955–61. https://doi.org/10.1002/cpt1973146955.
Miners JO, Birketc JO. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmaeol. 1998;45(6):525–38. https://doi.org/10.1046/j.1365-2125.1998.00721.x.
Ngui JS, Chen Q, Shou M, et al. In vitro stimulation of warfarin metabolism by quinidine: increases in the formation of 4′- and 10-hydroxywarfarin. Drug Metab Dispos. 2001;29(6):877–86.
Zhang Z, Fasco MJ, Huang Z, Guengerich FP, Kaminsky LS. Human cytochromes P4501A1 and P450 1A2: R-warfarin metabolism as a probe. Drug Metab Dispos. 1995;23(12):1339–46.
Wienkers LC, Wurden CJ, Storch E, et al. Formation of (R)-8-hydroxywarfarin in human liver microsomes. A new metabolic marker for the (S)-mephenytoin hydroxylase, P4502C19. Drug Metab Dispos. 1996;24(5):610–4.
Ye H, Sui D, Liu W, Yuan Y, Ouyang Z, Wei Y. Effects of CYP2C11 gene knockout on the pharmacokinetics and pharmacodynamics of warfarin in rats. Xenobiotica. 2019;49(12):1478–84. https://doi.org/10.1080/00498254.2019.1579006.
Jian-mei Wu, Zhang M-S, Zhang J-H. Pharmacokinetics of Panax notoginseng on warfarin in rats. Chin J Clin Pharmacol. 2018;34(04):461–3.
Jiang S, Peng X-S, Huang Z-H, et al. Compatibility of astragalus polysaccharides and notoginseng total saponin in diabetic rat kidney IV collagen type and the influence of layer adhesion protein expression. J Hunan Univ Chin Med. 2015;35(12):18–22.
Han SY, Li HX, Ma X, et al. Evaluation of the antimyocardial ischemia effect of individual and combined extracts of Panax notoginseng and Carthamus tinctorius in rats. J Ethnopharmacol. 2013;145(3):722–7.
Liu R, Qin M, Hang P, et al. Effects of Panax notoginseng saponins on the activities of CYP1A2, CYP2C9, CYP2D6 and CYP3A4 in rats in vivo. Phytother Res. 2012;26(8):1113–8.
Xiao-yu L. Study on the expression of CYP450 enzyme in rat liver mediated by total saponins of Panax notoginseng and its mechanism. Shanghai: Shanghai Jiao Tong University; 2009.
Mo Q-Y, Zu-e Lu. Clinical application effect and pharmacological analysis of Panax notoginseng. J Gen Stomatol. 2018;5(21):110–1.
Jin Z, Gao L, Zhang L, et al. Antimicrobial activity of saponins produced by two novel endophytic fungi from Panax notoginseng. Nat Prod Res. 2017;31(22):2700–3. https://doi.org/10.1080/14786419.2017.1292265.
Kitamura K, Takamura Y, Iwamoto T, et al. Dammarane-type triterpene extracts of Panax notoginseng root ameliorates hyperglycemia and insulin sensitivity by enhancing glucose uptake in skeletal muscle. Biosci Biotechnol Biochem. 2017;81(2):335–42. https://doi.org/10.1080/09168451.2016.1246173.
Liao J, Wei B, Chen H, et al. Bioinformatics investigation of therapeutic mechanisms of Xuesaitong capsule treating ischemic cerebrovascular rat model with comparative transcriptome analysis. Am J Transl Res. 2016;8(5):2438–49.
Ratno Budiarto B, Chan WH. Oxidative stresses-mediated apoptotic effects of ginsenoside rb1 on pre- and post-implantation mouse embryos in vitro and in vivo. Environ Toxicol. 2017;32(8):1990–2003. https://doi.org/10.1002/tox.22366.
Tian XP, Zhao HM, Xue MJ. Effect of xuesaitong on elderly patients with severe acute pancreatitis by selective arterial intervention therapy. Biomed Res-India. 2017;28(12):5233–6.
Xu D, Huang P, Yu Z, et al. Efficacy and safety of Panax notoginseng saponin therapy for acute intracerebral hemorrhage, meta-analysis, and mini review of potential mechanisms of action. Front Neurol. 2014;5:274. https://doi.org/10.3389/fneur.2014.00274.
Yang M, Liang EY, Mao LJ, et al. Notoginsenoside R1 has thrombopoietic effect in irradiated mice. Blood. 2016;128(22):4932.
Yang Z-M, Yang X-F. Inhibitory effect of Panax notoginseng saponins on drug metabolizing enzyme CYP3A in rat liver and its kinetic analysis. Chin J Tradit Chin Med. 2012;37(22):3486–9.
Lin Y, Wei Y, Hu X, et al. Evaluation of lentinan effects on cytochrome P450 activity in rats by a cocktail method. Iran J Basic Med Sci. 2019;22(3):296–301. https://doi.org/10.22038/ijbms.2019.31611.7611.
Shimada T, Yamazaki H, Mimura M, et al. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994;270:414–23.
Niwa T, Morimoto M, Hirai T, et al. Effect of penicillin-based antibiotics, amoxicillin, ampicillin, and piperacillin, on drug-metabolizing activities of human hepatic cytochromes P450. J Toxicol Sci. 2016;41(1):143–6. https://doi.org/10.2131/jts.41.143.
Han W, Shao Fu, Fang X-L. Study on oral absorption and pharmacokinetics of Ginsenoside Rg1 and Rb1 in Panax notoginseng saponins. J Pharm Sci. 2007;42(8):849–53.
Acknowledgements
The authors wish to thank Mrs. Tingting Wu regarding the collection of the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Fujian Provincial Health Technology Project, China (2019-CX-19).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Code availability
Not applicable.
Author contributions
WJC, JMW, and JFQ, who participated in the design and conduct of the study, contributed equally to the article. MNL, SJJ, ZWZ, ZWF, and MRC analyzed the data and conducted the section on statistical analysis, and JHZ revised the manuscript. All the authors contributed to the final paper.
Ethical approval
The protocol of the study was reviewed and approved by the Ethics Committee of Fujian Medical University. All institutional and national guidelines for the care of the laboratory animals were followed.
Consent for publication
Not applicable.
Consent for publication
Not applicable.
Rights and permissions
About this article
Cite this article
Qian, J., Chen, W., Wu, J. et al. Effects and Mechanism of Action of Panax notoginseng Saponins on the Pharmacokinetics of Warfarin. Eur J Drug Metab Pharmacokinet 47, 331–342 (2022). https://doi.org/10.1007/s13318-022-00753-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00753-0