Abstract
Background and Objectives
Previous studies have revealed that sulfation, as mediated by the estrogen-sulfating cytosolic sulfotransferase (SULT) SULT1E1, is involved in the metabolism of 17β-estradiol (E2), 4-hydroxytamoxifen (4OH-tamoxifen), and diethylstilbestrol in humans. It is an interesting question whether the genetic polymorphisms of SULT1E1, the gene that encodes the SULT1E1 enzyme, may impact on the metabolism of E2 and these two drug compounds through sulfation.
Methods
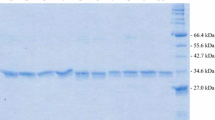
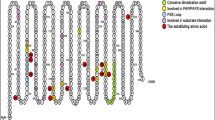
In this study, five missense coding single nucleotide polymorphisms of the SULT1E1 gene were selected to investigate the sulfating activity of the coded SULT1E1 allozymes toward E2, 4OH-tamoxifen, and diethylstilbestrol. Corresponding cDNAs were generated by site-directed mutagenesis, and recombinant SULT1E1 allozymes were bacterially expressed, affinity-purified, and characterized using enzymatic assays.
Results
Purified SULT1E1 allozymes were shown to display differential sulfating activities toward E2, 4OH-tamoxifen, and diethylstilbestrol. Kinetic analysis revealed further distinct Km (reflecting substrate affinity) and Vmax (reflecting catalytic activity) values of the five SULT1E1 allozymes with E2, 4OH-tamoxifen, and diethylstilbestrol as substrates.
Conclusions
Taken together, these findings highlighted the significant differences in E2-, as well as the drug-sulfating activities of SULT1E1 allozymes, which may have implications in the differential metabolism of E2, 4OH-tamoxifen, and diethylstilbestrol in individuals with different SULT1E1 genotypes.









Similar content being viewed by others
References
Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998;339:1609–18. https://doi.org/10.1056/NEJM199811263392207.
Crewe HK, Ellis SW, Lennard MS, Tucker GT. Variable contribution of cytochromes P450 2D6, 2C9 and 3A4 to the 4-hydroxylation of tamoxifen by human liver microsomes. Biochem Pharmacol. 1997;53(2):171–8. https://doi.org/10.1016/s0006-2952(96)00650-8.
Crewe HK, Notley LM, Wunsch RM, Lennard MS, Gillam EM. Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4′-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug Metab Dispos. 2002;30(8):869–74. https://doi.org/10.1124/dmd.30.8.869.
Jordan VC, Collins MM, Rowsby L, Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977;75(2):305–16. https://doi.org/10.1677/joe.0.0750305.
Chang M. Tamoxifen resistance in breast cancer. Biomol Ther (Seoul). 2012;20(3):256–67. https://doi.org/10.4062/biomolther.2012.20.3.256.
Iwase H, Yamamoto Y. Clinical benefit of sequential use of endocrine therapies for metastatic breast cancer. Int J Clin Oncol. 2015;20:253–61. https://doi.org/10.1007/s10147-015-0793-8.
Lonning PE, Taylor PD, Anker G, Iddon J, Wie L, Jorgensen LM, Mella O, Howell A. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001;67:111–6. https://doi.org/10.1023/a:1010619225209.
Mahtani RL, Stein A, Vogel CL. High-dose estrogen as salvage hormonal therapy for highly refractory metastatic breast cancer: a retrospective chart review. Clin Ther. 2009;31:2371–8. https://doi.org/10.1016/j.clinthera.2009.11.002.
de Voogt HJ, Smith PH, Pavone-Macaluso M, de Pauw M, Suciu S. Cardiovascular side effects of diethylstilbestrol, cyproterone acetate, medroxyprogesterone acetate and estramustine phosphate used for the treatment of advanced prostatic cancer: results from European Organization for Research on Treatment of Cancer trials 30761 and 30762. J Urol. 1986;135(2):303–7. https://doi.org/10.1016/s0022-5347(17)45620-5.
Fisher B, Costantino JP, Redmond CK, Fisher ER, Wickerham DL, Cronin WM. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994;86(7):527–37. https://doi.org/10.1093/jnci/86.7.527.
Latifyan S, Vansteelandt C, Lecomte S, Efira A. Tamoxifen induced thromboembolic events in breast cancer. Some possible mechanisms. Rev Med Brux. 2017;38(6):494–500.
Manikandan R, Srirangam SJ, Pearson E, Brown SCW, O'Reilly P, Collins GN. Diethylstilboestrol versus bicalutamide in hormone refractory prostate carcinoma: a prospective randomized trial. Urol Int. 2005;75(3):217–21. https://doi.org/10.1159/000087797.
Nayfield SG, Gorin MB. Tamoxifen-associated eye disease. A review. J Clin Oncol. 1996;14(3):1018–26. https://doi.org/10.1200/JCO.1996.14.3.1018.
Salomao SR, Watanabe SE, Berezovsky A, Motono M. Multifocal electroretinography, color discrimination and ocular toxicity in tamoxifen use. Curr Eye Res. 2007;32(4):345–52. https://doi.org/10.1080/02713680701229638.
Smith DC, Redman BG, Flaherty LE, Li L, Strawderman M, Pienta KJ. A phase II trial of oral diethylstilbesterol as a second-line hormonal agent in advanced prostate cancer. Urology. 1998;52(2):257–60. https://doi.org/10.1016/s0090-4295(98)00173-3.
Falany JL, Pilloff DE, Leyh TS, Falany CN. Sulfation of raloxifene and 4-hydroxytamoxifen by human cytosolic sulfotransferases. Drug Metab Dispos. 2006;34(3):361–8. https://doi.org/10.1124/dmd.105.006551.
Nishiyama T, Ogura K, Nakano H, Ohnuma T, Kaku T, Hiratsuka A, Muro K, Watabe T. Reverse geometrical selectivity in glucuronidation and sulfation of cis- and trans-4-hydroxytamoxifens by human liver UDP-glucuronosyltransferases and sulfotransferases. Biochem Pharmacol. 2002;63(10):1817–30. https://doi.org/10.1016/s0006-2952(02)00994-2.
Suiko M, Sakakibara Y, Liu MC. Sulfation of environmental estrogen-like chemicals by human cytosolic sulfotransferases. Biochem Biophys Res Commun. 2000;267(1):80–4. https://doi.org/10.1006/bbrc.1999.1935.
Falany C, Roth JA. Properties of human cytosolic sulfotransferases involved in drug metabolism. In: Jeffery EH, editor. Human drug metabolism; from molecular biology to man. Boca Raton: CRC; 1993. p. 101–115.
Mulder GJ, Jakoby WB. Sulfation in conjugation reactions. In: Mulder GJ, Jakoby WB, editors. Drug metabolism. London: Taylor and Francis; 1990. p. 107–161.
Weinshilboum R, Otterness D. Sulfotransferase enzymes. In: Kaufmann FC, editor. Conjugation-deconjugation reactions in drug metabolism and toxicity. Berlin: Springer; 1994. p. 45–78.
Blanchard RL, Freimuth RR, Buck J, Weinshilboum RM, Coughtrie MW. A proposed nomenclature system for the cytosolic sulfotransferase (SULT) superfamily. Pharmacogenetics. 2004;14(3):199–21111. https://doi.org/10.1097/00008571-200403000-00009.
Freimuth RR, Wiepert M, Chute CG, Wieben ED, Weinshilboum RM. Human cytosolic sulfotransferase database mining: identification of seven novel genes and pseudogenes. Pharmacogenomics J. 2004;4(1):54–655. https://doi.org/10.1038/sj.tpj.6500223.
Falany CN, Krasnykh V, Falany JL. Bacterial expression and characterization of a cDNA for human liver estrogen sulfotransferase. J Steroid Biochem Mol Biol. 1995;52(6):529–39.
Zhang H, Varlamova O, Vargas FM, et al. Sulfuryl transfer: the catalytic mechanism of human estrogen sulfotransferase. J Biol Chem. 1998;273(18):10888–92.
Petrotchenko EV, Doerflein ME, Kakuta Y, et al. Substrate gating confers steroid specificity to estrogen sulfotransferase. J Biol Chem. 1999;274(42):30019–22.
Agarwal N, Alex AB, Farnham JM, Patel S, Gill D, Buckley TH, Stephenson RA, Cannon-Albright L. Inherited variants in SULT1E1 and response to abiraterone acetate by men with metastatic castration refractory prostate cancer. J Urol. 2016;196(4):1112–6. https://doi.org/10.1016/j.juro.2016.04.079.
Choi JY, Lee KM, Park SK, Noh DY, Ahn SH, Chung HW, Han W, Kim JS, Shin SG, Jang IJ, Yoo KY, Hirvonen A, Kang D. Genetic polymorphisms of SULT1A1 and SULT1E1 and the risk and survival of breast cancer. Cancer Epidemiol Biomark Prev. 2005;14(5):1090–5. https://doi.org/10.1158/1055-9965.EPI-04-0688.
Cohen S, Laitman Y, Kaufman B, Milgrom R, Nir U, Friedman E. SULT1E1 and ID2 genes as candidates for inherited predisposition to breast and ovarian cancer in Jewish women. Fam Cancer. 2009;8(2):135–44. https://doi.org/10.1007/s10689-008-9218-4.
Daniels J, Kadlubar S. Sulfotransferase genetic variation: from cancer risk to treatment response. Drug Metab Rev. 2013;45(4):415–22. https://doi.org/10.3109/03602532.2013.835621.
Hirata H, Hinoda Y, Okayama N, Suehiro Y, Kawamoto K, Kikuno N, Rabban JT, Chen LM, Dahiya R. CYP1A1, SULT1A1, and SULT1E1 polymorphisms are risk factors for endometrial cancer susceptibility. Cancer. 2008;112(9):1964–73. https://doi.org/10.1002/cncr.23392.
Rebbeck TR, Troxel AB, Wang Y, Walker AH, Panossian S, Gallagher S, Shatalova EG, Blanchard R, Bunin G, DeMichele A, Rubin SC, Baumgarten M, Berlin M, Schinnar R, Berlin JA, Strom BL. Estrogen sulfation genes, hormone replacement therapy, and endometrial cancer risk. J Natl Cancer Inst. 2006;98(18):1311–20. https://doi.org/10.1093/jnci/djj360.
Woo HI, Lee SK, Kim J, Kim SW, Yu J, Bae SY, Lee JE, Nam SJ, Lee SY. Variations in plasma concentrations of tamoxifen metabolites and the effects of genetic polymorphisms on tamoxifen metabolism in Korean patients with breast cancer. Oncotarget. 2017;8(59):100296–311. https://doi.org/10.18632/oncotarget.22220.
Yanagisawa K, Sakakibara Y, Suiko M, Takami Y, Nakayama T, Nakajima H, Takayanagi K, Natori Y, Liu MC. cDNA cloning, expression, and characterization of the human bifunctional ATP sulfurylase/adenosine 5′-phosphosulfate kinase enzyme. Biosci Biotechnol Biochem. 1998;62(5):1037–40. https://doi.org/10.1271/bbb.62.1037.
Hui Y, Liu MC. Sulfation of ritodrine by the human cytosolic sulfotransferases (SULTs): Effects of SULT1A3 genetic polymorphism. Eur J Pharmacol. 2015;761:125–9. https://doi.org/10.1016/j.ejphar.2015.04.039.
Dunbrack RL Jr. Rotamer libraries in the 21st century. Curr Opin Struct Biol. 2002;12(4):431–40. https://doi.org/10.1016/S0959-440X(02)00344-5.
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–12. https://doi.org/10.1002/jcc.20084.
Mansel R, Goyal A, Nestour EL, Masini-Eteve V, O'Connell K. Afimoxifene Breast Pain Research Group, A phase II trial of Afimoxifene (4-hydroxytamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat. 2007;106(3):389–97. https://doi.org/10.1007/s10549-007-9507-x.
Turo R, Tan K, Thygesen H, Sundaram SK, Chahal R, Prescott S, Cross WR. Diethylstilboestrol (1 mg) in the management of castration-resistant prostate cancer. Urol Int. 2015;94(3):307–12. https://doi.org/10.1159/000365198.
Falany JL, Falany CN. Expression of cytosolic sulfotransferases in normal mammary epithelial cells and breast cancer cell lines. Cancer Res. 1996;56(7):1551–5.
Nakamura Y, Suzuki T, Fukuda T, Ito A, Endo M, Moriya T, Arai Y, Sasano H. Steroid sulfatase and estrogen sulfotransferase in human prostate cancer. Prostate. 2006;66(9):1005–122. https://doi.org/10.1002/pros.20426.
Qian Y, Deng C, Song WC. Expression of estrogen sulfotransferase in MCF-7 cells by cDNA transfection suppresses the estrogen response: potential role of the enzyme in regulating estrogen-dependent growth of breast epithelial cells. J Pharmacol Exp Ther. 1998;286(1):555–60.
Hui Y, Luo L, Zhang L, Kurogi K, Zhou C, Sakakibara Y, Suiko M, Liu MC. Sulfation of afimoxifene, endoxifen, raloxifene, and fulvestrant by the human cytosolic sulfotransferases (SULTs): a systematic analysis. J Pharmacol Sci. 2015;128(3):144–9. https://doi.org/10.1016/j.jphs.2015.06.004.
Areepium N, Panomvana D, Rungwanonchai P, Sathaporn S, Voravud N. Effects of CYP2D6 and UGT2B7 polymorphisms on pharmacokinetics of tamoxifen in Thai breast cancer patients. Breast Cancer (Dove Med Press). 2013;5:73–8. https://doi.org/10.2147/BCTT.S47172.
de Vries Schultink AH, Zwart W, Linn SC, Beijnen JH, Huitema AD. Effects of pharmacogenetics on the pharmacokinetics and pharmacodynamics of tamoxifen. Clin Pharmacokinet. 2015;54(8):797–810. https://doi.org/10.1007/s40262-015-0273-3.
Fernandez-Santander A, Gaibar M, Novillo A, Romero-Lorca A, Rubio M, Chicharro LM, Tejerina A, Bandres F. Relationship between genotypes Sult1a2 and Cyp2d6 and tamoxifen metabolism in breast cancer patients. PLoS ONE. 2013;8(7):e70183. https://doi.org/10.1371/journal.pone.0070183.
Gjerde J, Hauglid M, Breilid H, Lundgren S, Varhaug JE, Kisanga ER, Mellgren G, Steen VM, Lien EA. Effects of CYP2D6 and SULT1A1 genotypes including SULT1A1 gene copy number on tamoxifen metabolism. Ann Oncol. 2008;19(1):56–61. https://doi.org/10.1093/annonc/mdm434.
Lim JS, Chen XA, Singh O, Yap YS, Ng RC, Wong NS, Wong M, Lee EJ, Chowbay B. Impact of CYP2D6, CYP3A5, CYP2C9 and CYP2C19 polymorphisms on tamoxifen pharmacokinetics in Asian breast cancer patients. Br J Clin Pharmacol. 2011;71(5):737–50. https://doi.org/10.1111/j.1365-2125.2011.03905.x.
Murdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G, Simon W, Fasching PA, Fehm T, German Tamoxifen, and AI Clinician Group, Eichelbaum M, Schwab M, Brauch H. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89(5):708–17. https://doi.org/10.1038/clpt.2011.27.
Zafra-Ceres M, de Haro T, Farez-Vidal E, Blancas I, Bandres F, de Duenas EM, Ochoa-Aranda E, Gomez-Capilla JA, Gomez-Llorente C. Influence of CYP2D6 polymorphisms on serum levels of tamoxifen metabolites in Spanish women with breast cancer. Int J Med Sci. 2013;10(7):932–7. https://doi.org/10.7150/ijms.5708.
Adjei AA, Thomae BA, Prondzinski JL, Eckloff BW, Wieben ED, Weinshilboum RM. Human estrogen sulfotransferase (SULT1E1) pharmacogenomics: gene resequencing and functional genomics. Br J Pharmacol. 2003;139(8):1373–82. https://doi.org/10.1038/sj.bjp.0705369.
Pedersen LC, Petrotchenko E, Shevtsov S, Negishi M. Crystal structure of the human estrogen sulfotransferase-PAPS complex: evidence for catalytic role of Ser137 in the sulfuryl transfer reaction. J Biol Chem. 2002;277(20):17928–32. https://doi.org/10.1074/jbc.M111651200.
Shevtsov S, Petrotchenko EV, Pedersen LC, Negishi M. Crystallographic analysis of a hydroxylated polychlorinated biphenyl (OH-PCB) bound to the catalytic estrogen binding site of human estrogen sulfotransferase. Environ Health Perspect. 2003;111(7):884–8. https://doi.org/10.1289/ehp.6056.
Thomas MP, Potter BV. The structural biology of oestrogen metabolism. J Steroid Biochem Mol Biol. 2013;137:27–49. https://doi.org/10.1016/j.jsbmb.2012.12.014.
Kakuta Y, Pedersen LG, Carter CW, Negishi M, Pedersen LC. Crystal structure of estrogen sulphotransferase. Nat Struct Biol. 1997;4(11):904–8. https://doi.org/10.1038/nsb1197-904.
Petrotchenko EV, Pedersen LC, Borchers CH, Tomer KB, Negishi M. The dimerization motif of cytosolic sulfotransferases. FEBS Lett. 2001;490:39–433. https://doi.org/10.1016/s0014-5793(01)02129-9.
Cook I, Wang T, Almo SC, Kim J, Falany CN, Leyh TS. The gate that governs sulfotransferase selectivity. Biochemistry. 2012;52(2):415–24. https://doi.org/10.1021/bi301492j.
Tibbs ZE, Rohn-Glowacki KJ, Crittenden F, Guidry AL, Falany CN. Structural plasticity in the human cytosolic sulfotransferase dimer and its role in substrate selectivity and catalysis. Drug Metab Pharmacokinet. 2015;30(1):3–20. https://doi.org/10.1016/j.dmpk.2014.10.004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported in part by a grant from National Institutes of Health (Grant # R03HD071146).
Conflicts of Interest
The authors (A.E., F. A., M.A., A.B., M.R., K.K., and M.L.) declare no conflicts of interest.
Ethics Approval
N/A.
Availability of Data and Material
Data and materials will be made available upon request.
Code Availability
N/A.
Consent to Participate
N/A.
Consent for Publication
All authors have consented to the publication of this manuscript. No permissions from other parties are required.
Author Contributions
AE, FA, MA, AB, and MR performed experiments (cDNA cloning, recombinant protein expression/purification, and enzymatic characterization) and analyzed data. AE, KK, and ML analyzed data and wrote the manuscript. ML devised the project.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
El Daibani, A.A., Alherz, F.A., Abunnaja, M.S. et al. Impact of Human SULT1E1 Polymorphisms on the Sulfation of 17β-Estradiol, 4-Hydroxytamoxifen, and Diethylstilbestrol by SULT1E1 Allozymes. Eur J Drug Metab Pharmacokinet 46, 105–118 (2021). https://doi.org/10.1007/s13318-020-00653-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-020-00653-1