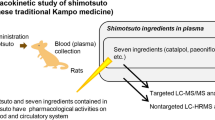
Abstract
Background and Objective
Danggui-Shaoyao-San (DSS), a famous Chinese formula, has been widely used to treat gynecological disorders since ancient times and has recently showed efficacy in treating Alzheimer’s disease. Butylidenephthalide (BDPH) and alisol B (ALI) are recognized as the primary active ingredients of DSS. The objectives of the present study were to evaluate the pharmacokinetic comparative study of BDPH and ALI in herbal extracts and their purified forms.
Method
A sensitive and specific high-performance liquid chromatography tandem mass spectrometry (HPLC–MS/MS) methodology was developed to determine the concentration level of BDPH and ALI in rat plasma. This approach enables a real-time pharmacokinetics profiling of BDPH and ALI in DSS extracts as well as their purified forms in rats after oral administration.
Results
The validated method showed an evident linearity over a wide range of dosages (r > 0.99) with sensitivity down to 1.0 ng/mL for each analyte. The extraction recovery of the analyte ranged from 80.8 to 99.1%. The pharmacokinetic parameters were significantly different in herbal extracts and their purified forms. The results showed that the absorption of both BDPH and ALI from DSS extracts was significantly greater compared with their purified forms.
Conclusions
A highly specific, sensitive and rapid HPLC–MS/MS method was developed and applied for the determination of BDPH and ALI in rat plasma. It was found that BDPH and ALI had higher bioavailability in the DSS extract compared with their purified forms.



Similar content being viewed by others
References
Xue T, Roy R. Studying traditional Chinese medicine. Science. 2003;300(5620):7401.
Wang Y, Li G, Zhou Y, et al. The difference between blood-associated and water-associated herbs of Danggui-Shaoyao San in theory of TCM, based on serum pharmacochemistry. Biomed Chromatogr. 2016;30(4):579–87.
Hatip-Al-Khatib I, Egashira N, Mishima K, et al. Determination of the effectiveness of components of the herbal medicine Toki-Shakuyaku-San and fractions of Angelica acutiloba in improving the scopolamine-induced impairment of rat’s spatial cognition in eight-armed radial maze test. J Pharmacol Sci. 2004;96(1):33–41.
Hu ZY, Liu G, Yuan H, et al. Danggui-Shaoyao-San and its active fraction JD-30 improve Aβ-induced spatial recognition deficits in mice. J Ethnopharmacol. 2010;128(2):365–72.
Lan Z, Liu J, Chen L, et al. Danggui-Shaoyao-San ameliorates cognition deficits and attenuates oxidative stress-related neuronal apoptosis in d-galactose-induced senescent mice. J Ethnopharmacol. 2012;141(1):386–95.
Luo Y, Wang Q, Zhang Y. A systems pharmacology approach to decipher the mechanism of Danggui-Shaoyao-San decoction for the treatment of neurodegenerative diseases. J Ethnopharmacol. 2016;178:66–81.
Chen L, Qi J, Chang YX, et al. Identification and determination of the major constituents in Traditional Chinese Medicinal formula Danggui-Shaoyao-San by HPLC-DAD-ESI-MS/MS. J Pharm Biomed Anal. 2009;50(2):127–37.
Chan S, Jiang Y, Jiang Z, et al. Phthalides, instead of ferulic acid and tetramethylpyrazine, are the appropriate bioactive chemical markers for the quality assessment and pharmacological evaluation of Angelica sinensis and Ligusticum chuanxiong. In: Singh VK, Govil JN, editors. Recent progress in medicinal plants Vol. 23, phytopharmacology and therapeutic values V. Houston: Studium Press; 2007.
Ko WC, Chang LD, Wang GY, et al. Pharmacological effects of butylidenephthalide. Phytotherapy Res. 1994;8(6):321–6.
Ko WC, Sheu JR, Tzeng SH, et al. The selective antianginal effect without changing blood pressure of butylidenephthalide in conscious rats. Planta Med. 1998;64(3):229–32.
Lin G, Chan SS, Chung HS, et al. Chemistry and biological activities of naturally occurring phthalides. Stud Nat Prod Chem. 2005;32:611–69.
Teng CM, Chen WY, Ko WC, et al. Antiplatelet effect of butylidenephthalide. Biochimica et Biophysica Acta (BBA) Gen Subj. 1987;924(3):375–82.
Xiu RJ. Microcirculation and traditional Chinese medicine. JAMA. 1988;260(12):1755–7.
Koyama T. Osteoporosis with Kampo treatment. Sanfujinka Chiryo. 1991;63:203–7.
Huang YT, Huang DM, Chueh SC, et al. Alisol B acetate, a triterpene from Alismatis rhizoma, induces Bax nuclear translocation and apoptosis in human hormone-resistant prostate cancer PC-3 cells. Cancer Lett. 2006;231(2):270–8.
Chen HW, Hsu MJ, Chien CT, et al. Effect of alisol B acetate, a plant triterpene, on apoptosis in vascular smooth muscle cells and lymphocytes. Eur J Pharmacol. 2001;419(2):127–38.
Xu YH, Zhao LJ, Li Y. Alisol B acetate induces apoptosis of SGC7901 cells via mitochondrial and phosphatidylinositol 3-kinases/Akt signaling pathways. World J Gastroenterol WJG. 2009;15(23):2870.
Matsuda H, Kageura T, Toguchida I, et al. Effects of sesquiterpenes and triterpenes from the rhizome of Alisma orientale on nitric oxide production in lipopolysaccharide-activated macrophages: absolute stereostructures of alismaketones-B 23-acetate and-C 23-acetate. Bioorg Med Chem Lett. 1999;9(21):3081–6.
Lee SM, Kim JH, Zhang Y, et al. Anti-complementary activity of protostane-type triterpenes from Alismatis rhizoma. Arch Pharmacal Res. 2003;26(6):463–5.
Kubo M, Matsuda H, Tomohiro N, et al. Studies on Alismatis rhizoma. I. Anti-allergic effects of methanol extract and six terpene components from Alismatis rhizoma (dried rhizome of Alisma orientale). Biol Pharm Bull. 1997;20(5):511–6.
Lee JW, Kobayashi Y, Nakamichi Y, et al. Alisol-B, a novel phyto-steroid, suppresses the RANKL-induced osteoclast formation and prevents bone loss in mice. Biochem Pharmacol. 2010;80(3):352–61.
Xu S, Peng J, Li Y, et al. Pharmacokinetic comparisons of rutaecarpine and evodiamine after oral administration of Wu-Chu-Yu extracts with different purities to rats. J Ethnopharmacol. 2012;139(2):395–400.
Xiao F, Li Q, Liang K, et al. Comparative pharmacokinetics of three triterpene acids in rat plasma after oral administration of Poria extract and its formulated herbal preparation: GuiZhi-FuLing capsule. Fitoterapia. 2012;83(1):117–24.
Chen LL, Wang YH, Jin Q, et al. Identification and determination of absorbed components of Danggui-Shaoyao-San in rat plasma. Chin J Nat Med. 2011;9(5):363–8.
Yan R, Ling Ko N, Ma B, et al. Metabolic conversion from co-existing ingredient leading to significant systemic exposure of Z-butylidenephthalide, a minor ingredient in Chuanxiong Rhizoma in rats. Curr Drug Metab. 2012;13(5):524–34.
Feng B, Zhang J, Chen G, et al. Effect of different compatibility of tetramethylpyrazine on its pharmacokinetics in vivo. China J Chin Mater Med. 2012;37(9):1311–4 (in Chinese).
Feng B, Zhang J, Chen G, et al. Comparative study on pharmacokinetics of tetramethylpyrazine, ferulic acid and their compatibility. China J Chin Mater Med. 2010;35(7):900–3 (in Chinese).
Zhang L, Yan R, Su R, et al. Bioavailability enhancement of osthole after oral administration of Bushen Yizhi prescription extract to rats followed by Cnidium monnieri (L.) Cusson fruits extract in comparison to pure osthole at different doses. J Ethnopharmacol. 2014;152(2):266–71.
Qian M, Shi L, Hu J. Enzyme kinetics of ligustilide metabolism in rat liver microsomes. Acta Pharm Sin. 2009;44(4):395–400 (in Chinese).
Gao LN, Zhang Y, Cui YL, et al. Comparison of paeoniflorin and albiflorin on human CYP3A4 and CYP2D6. Evid Based Complement Altern Med. 2015;2015(9):470219.
He WQ, Lv WS, Zhang Y, et al. Study on pharmacokinetics of three preparations from levistolide A by LC-MS-MS. J Chromatogr Sci. 2015;53(8):1265–73.
Zhao HR, Feng SX. Pharmacokinetics of butylidene phthalide in the volatile oil from Angelica sinensis(Oliv.) Diels in rabbits. West China J Pharm Sci. 2009;24(2):162–4 (in Chinese).
Yan R, Ko NL, Li SL, et al. Pharmacokinetics and metabolism of ligustilide, a major bioactive component in Rhizoma Chuanxiong, in the rat. Drug Metab Dispos Biol Fate Chem. 2008;36(2):400–8.
Thomas M, George NI, Saini UT, et al. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported by the National Natural Science Foundation of China (nos. 81473740, 81573638, 81673627, 81674040), the Natural Science Foundation of Guangdong Province (no. 2017A030313666) and Guangzhou Science Technology and Innovation Commission Technology Research Projects (no. 201805010005).
Conflict of interest
Hui-fei Wu, Xiang-yu Wang, Ji-feng Deng, Shi-jian Quan, Qi Wang and Wei-rong Li declare that they have no conflict of interest.
Ethical approval
The experimental design was approved by the Guangzhou University of Chinese Medicine Committee on Animal Care and Use, and the study was performed in accordance with the Internal Charter on the Humane Care and Use of Laboratory Animals.
Rights and permissions
About this article
Cite this article
Wu, Hf., Wang, Xy., Deng, Jf. et al. Pharmacokinetic Profiling of Butylidenephthalide and Alisol B in Danggui-Shaoyao-San in Rats. Eur J Drug Metab Pharmacokinet 43, 645–653 (2018). https://doi.org/10.1007/s13318-018-0476-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-018-0476-8