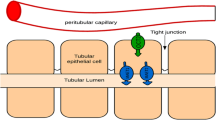
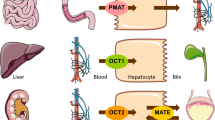
Abstract
Background and objectives
Physiologically based pharmacokinetic (PBPK) modelling and simulation enable researchers to overcome practical limitations for clinical trials on special populations. This study was conducted to investigate how the PBPK model describes the pharmacokinetics of metformin in young adult and elderly populations and to predict the pharmacokinetics of metformin in patients with renal or hepatic impairment in both populations.
Methods
A first-order absorption/PBPK model for metformin was built in the Simcyp simulator version 14 release 1. A full PBPK model was constructed for metformin based on physicochemical properties and clinical observations. The model was refined and validated using clinical plasma concentration data obtained in healthy young adults and elderly after the oral administration of metformin. Metformin pharmacokinetics in patients with renal or hepatic impairment were then investigated and compared by simulation.
Results
The PBPK model reasonably predicted the pharmacokinetic profiles of metformin for both young adults and the elderly. The predicted pharmacokinetic parameters, including maximum concentration, area under the time–concentration curve, and apparent oral clearance values, were within 1.5-fold of the observed data of metformin. In the simulation results, the systemic exposure of metformin was expected to be markedly increased not only with a decrease in renal function but also with severe hepatic impairments.
Conclusions
The PBPK model adequately characterised the pharmacokinetics of metformin in both young adult and elderly populations. PBPK modelling and simulation can be used as a useful tool to investigate and compare the pharmacokinetics in geriatric populations incorporating various disease conditions.



Similar content being viewed by others
References
Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73. doi:10.1146/annurev-pharmtox-010510-100540.
Jones HM, Chen Y, Gibson C, Heimbach T, Parrott N, Peters SA, et al. Physiologically based pharmacokinetic modeling in drug discovery and development: a pharmaceutical industry perspective. Clin Pharmacol Ther. 2015;97(3):247–62. doi:10.1002/cpt.37.
American Diabetes A. 7. Approaches to Glycemic Treatment. Diabetes Care. 2016;39(Suppl 1):S52–9. doi:10.2337/dc16-S010.
Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50(2):81–98. doi:10.2165/11534750-000000000-00000.
Kimura N, Okuda M, Inui K. Metformin transport by renal basolateral organic cation transporter hOCT2. Pharm Res. 2005;22(2):255–9.
Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007;74(2):359–71. doi:10.1016/j.bcp.2007.04.010.
Oh J, Chung H, Park SI, Yi SJ, Jang K, Kim AH, et al. Inhibition of the multidrug and toxin extrusion (MATE) transporter by pyrimethamine increases the plasma concentration of metformin but does not increase antihyperglycaemic activity in humans. Diabetes Obes Metab. 2016;18(1):104–8. doi:10.1111/dom.12577.
Grun B, Kiessling MK, Burhenne J, Riedel KD, Weiss J, Rauch G, et al. Trimethoprim–metformin interaction and its genetic modulation by OCT2 and MATE1 transporters. Br J Clin Pharmacol. 2013;76(5):787–96. doi:10.1111/bcp.12079.
Wang ZJ, Yin OQ, Tomlinson B, Chow MS. OCT2 polymorphisms and in vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genom. 2008;18(7):637–45. doi:10.1097/FPC.0b013e328302cd41.
FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. US Food and Drug Administration. 2016. http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed 13 Dec 2016.
Sambol NC, Chiang J, Lin ET, Goodman AM, Liu CY, Benet LZ, et al. Kidney function and age are both predictors of pharmacokinetics of metformin. J Clin Pharmacol. 1995;35(11):1094–102.
Jang K, Chung H, Yoon JS, Moon SJ, Yoon SH, Yu KS, et al. Pharmacokinetics, safety, and tolerability of metformin in healthy elderly subjects. J Clin Pharmacol. 2016;56(9):1104–10. doi:10.1002/jcph.699.
Li J, Guo HF, Liu C, Zhong Z, Liu L, Liu XD. Prediction of drug disposition in diabetic patients by means of a physiologically based pharmacokinetic model. Clin Pharmacokinet. 2015;54(2):179–93. doi:10.1007/s40262-014-0192-8.
Xia B, Heimbach T, Gollen R, Nanavati C, He H. A simplified PBPK modeling approach for prediction of pharmacokinetics of four primarily renally excreted and CYP3A metabolized compounds during pregnancy. AAPS J. 2013;15(4):1012–24. doi:10.1208/s12248-013-9505-3.
Burt HJ, Neuhoff S, Almond L, Gaohua L, Harwood MD, Jamei M, et al. Metformin and cimetidine: physiologically based pharmacokinetic modelling to investigate transporter mediated drug-drug interactions. Eur J Pharm Sci. 2016;88:70–82. doi:10.1016/j.ejps.2016.03.020.
Pentikainen PJ, Neuvonen PJ, Penttila A. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol. 1979;16(3):195–202.
Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981;12(2):235–46.
Jamei M, Bajot F, Neuhoff S, Barter Z, Yang J, Rostami-Hodjegan A, et al. A mechanistic framework for in vitro-in vivo extrapolation of liver membrane transporters: prediction of drug–drug interaction between rosuvastatin and cyclosporine. Clin Pharmacokinet. 2014;53(1):73–87. doi:10.1007/s40262-013-0097-y.
Neuhoff S, Yeo KR, Barter Z, Jamei M, Turner DB, Rostami-Hodjegan A. Application of permeability-limited physiologically-based pharmacokinetic models: part I-digoxin pharmacokinetics incorporating P-glycoprotein-mediated efflux. J Pharm Sci. 2013;102(9):3145–60. doi:10.1002/jps.23594.
Neuhoff S, Gaohua L, Burt H, Jamei M, Li L, Tucker GT, et al. Accounting for transporters in renal clearance: towards a mechanistic kidney model (Mech KiM). In: Sugiyama Y, Steffansen B, editors. Transporters in drug development: discovery, optimization, clinical study and regulation. New York: Springer New York; 2013. p. 155–77.
Rowland Yeo K, Aarabi M, Jamei M, Rostami-Hodjegan A. Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert Rev Clin Pharm. 2011;4(2):261–74. doi:10.1586/ecp.10.143.
Johnson TN, Boussery K, Rowland-Yeo K, Tucker GT, Rostami-Hodjegan A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet. 2010;49(3):189–206. doi:10.2165/11318160-000000000-00000.
Glucophage® Label. http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/020357s031,021202s016lbl.pdf. Accessed 11 Dec 2016.
Woitas RP, Flommersfeld S, Stoffel-Wagner B, Schiedermaier P, Brensing KA, Spengler U, et al. Renal functional reserve is well maintained in patients with advanced liver cirrhosis and ascites. Scand J Gastroenterol. 2002;37(11):1321–7.
Huang W, Lee SL, Yu LX. Mechanistic approaches to predicting oral drug absorption. AAPS J. 2009;11(2):217–24. doi:10.1208/s12248-009-9098-z.
Marathe PH, Wen Y, Norton J, Greene DS, Barbhaiya RH, Wilding IR. Effect of altered gastric emptying and gastrointestinal motility on metformin absorption. Br J Clin Pharmacol. 2000;50(4):325–32.
Ren J, Zhou Y, Zhang G, Zhou L, Zhao J, Wei Y, et al. Role of age-related decrease of renal organic cation transporter 2 in the effect of atenolol on renal excretion of metformin in rats. Eur J Drug Metab Pharmacokinet. 2015;40(3):349–54. doi:10.1007/s13318-014-0214-9.
Gaowa A, Motohashi H, Katsura T, Inui K. Effects of metabolic acidosis on expression levels of renal drug transporters. Pharm Res. 2011;28(5):1023–30. doi:10.1007/s11095-010-0348-7.
Komazawa H, Yamaguchi H, Hidaka K, Ogura J, Kobayashi M, Iseki K. Renal uptake of substrates for organic anion transporters Oat1 and Oat3 and organic cation transporters Oct1 and Oct2 is altered in rats with adenine-induced chronic renal failure. J Pharm Sci. 2013;102(3):1086–94. doi:10.1002/jps.23433.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant No. HI14C2770).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
SJR, HC, SJY, KSY and JYC declare no conflicts of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rhee, Sj., Chung, H., Yi, S. et al. Physiologically Based Pharmacokinetic Modelling and Prediction of Metformin Pharmacokinetics in Renal/Hepatic-Impaired Young Adults and Elderly Populations. Eur J Drug Metab Pharmacokinet 42, 973–980 (2017). https://doi.org/10.1007/s13318-017-0418-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-017-0418-x