Abstract
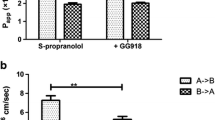
The study was to investigate the absorption mechanism and transport modulation of phillyrin by P-gp in Caco-2 cells and MDR1-MDCKII cells. Three concentrations of phillyrin were tested in transport studies. The absorptive transports of phillyrin in the two cell models were not concentration-dependent which indicated passive diffusion as the dominating process in the test concentrations. The absorptive P app were 7.15, 6.39 and 10.03 × 10−6 cm s−1, respectively, for different concentrations (2.2, 4.8 and 8.4 μg ml−1) in Caco-2 cells. And the low absorptive P app was consistent with the low oral bioavailability of phillyrin observed in pharmacokinetic experiments. In transport inhibition experiment, the efflux inhibitors, verapamil and GF120918 can increase the absorption of phillyrin in Caco-2 cells which suggested the involvement of efflux transporters. In the further inhibition experiment in MDR1-MDCKII cells, the absorption was greatly increased and the efflux of phillyrin was competitively inhibited by verapamil and GF120918, which confirmed the involvement of P-gp in the efflux of phillyrin.

Similar content being viewed by others
References
Acharya P, Tran TT, Polli JW et al (2006) P-gp expressed in a confluent monolayer of hMDR1-MDCKII cells has more than one efflux pathway with cooperative binding sites. Biochemistry 45:15505–15519. doi:10.1021/bi060593b
Ana MDL, María JAM, Lidia FM et al (2001) Lignan and phenylpropanoid glycosides from Phillyrea latifolia and their in vitro anti-inflammatory activity. Planta Med 67:219–223. doi:10.1055/s-2001-12004
Artursson P, Palm K, Luthman K (2001) Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev 46:27–43. doi:10.1016/S0169-409X(00)00128-9
Bentz J, Tran TT, Polli JW et al (2005) The steady-state Michaelis–Menten analysis of P-glycoprotein mediated transport through a confluent cell monolayer cannot predict the correct Michaelis constant K. Pharmacol Res 22:1667–1677. doi:10.1007/s11095-005-6627-z
Chen XF, Zhang JY, Xue CX et al (2004) Simultaneous determination of some active ingredients in anti-viral preparations of traditional Chinese medicine by micellar electrokinetic chromatography. Biomed Chromatogr 18:673–680. doi:10.1002/bmc.373
Cheng KC, Li C (2008) )Prediction of oral drug absorption in humans—from cultured cell lines and experimental animals. Expert Opin Drug Metab Toxicol 4:581–590. doi:10.1517/17425255.4.5.581
Dahan A, Amidon GL (2009) Grapefruit juice and its constituents augment colchicine intestinal absorption: potential hazardous interaction and the role of p-glycoprotein. Pharm Res 26:883–892. doi:10.1007/s11095-008-9789-7
Delie F, Rubas W (1997) A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Crit Rev Ther Drug Carrier Syst 14:221–286
Hidalgo IJ, Raub TJ, Borchardt RT (1989) Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–749
Hye HY, Mijin L, Min WL et al (2007) Effects of Schisandra Lignans on P-glycoprotein-mediated drug efflux in human intestinal Caco-2 Cells. Planta Med 73(5):444–450. doi:10.1055/s-2007-967178
Jeffrey EE, Kenneth RB, Patrick JM (2002) GF120918, a P-glycoprotein modulator, increases the concentration of unbound amprenavir in the central nervous system in rats. Antimicrob Agents Chemother 46(7):2284–2286. doi:10.1128/AAC.46.7.2284-2286.2002
Kim WY, Benet LZ (2004) P-glycoprotein (P-gp/MDR1)-mediated efflux of sex-steroid hormones and modulation of P-gp expression in vitro. Pharm Res 21(7):1284–1293. doi:10.1023/B:PHAM.0000033017.52484.81
Konsoula R, Barile FA (2005) Correlation of in vitro cytotoxicity with paracellular permeability in Caco-2 cells. Toxicol in Vitro 19:675–684. doi:10.1016/j.tiv.2005.03.006
Konsoula Z, Jung M (2009) Involvement of P-glycoprotein and multidrug resistance associated protein 1 on the transepithelial transport of a mercaptoacetamide-based histone-deacetylase inhibitor in Caco-2 cells. Biol Pharm Bull 32:74–78. doi:10.1248/bpb.32.74
Konsoula R, Mira J (2008) In vitro plasma stability, permeability and solubility of mercaptoacetamide histone deacetylase inhibitors. Int J Pharm 361:19–25. doi:10.1016/j.ijpharm.2008.05.001
Lan K, He JL, Tian Y et al (2008) Intra-herb pharmacokinetics interaction between quercetin and isorhamentin. Acta Pharmacol Sin 29:1376–1382. doi:10.1111/j.1745-7254.2008.00884.x
Lau YY, Okochi H, Huang Y et al (2006) Multiple transporters affect the disposition of atorvastatin and its two active hydroxy metabolites: application of in vitro and ex situ systems. J Pharmacol Exp Ther 316:762–771. doi:10.1124/jpet.105.093088
Li YX, Jiang XH, Zhou J (2005) The absorption mechanism of phillyrin in digestive tract in rat. West China J Pharm Sci 5:387–390. doi:CNKI:SUN:HXYO.0.2005-05-005
Maeng HJ, Yoo HJ, Kim IW et al (2002) P-glycoprotein-mediated transport of berberine across Caco-2 cell monolayers. J Pharm Sci 91:2614–2621. doi:10.1002/jps.10268
Meaney C, O’Driscoll C (1999) Mucus as a barrier to the permeability of hydrophilic and lipophilic compounds in the absence and presence of sodium taurocholate micellar systems using cell culture models. Eur J Pharm Sci 8:167–175. doi:10.1016/S0928-0987(99)00007-X
Nishibe S, Okabe K, Tsukamoto H et al (1982) Studies on the Chinese crude drug “Forsythiae Fructus”. VI. The structure and antibacterial activity of suspensaside isolated from Forsythia suspensa. Chem Pharm Bull 30:4548–4553
Perloff MD, Störmer E, Von Moltke LL et al (2003) Rapid assessment of P-glycoprotein inhibition and induction in vitro. Pharm Res 20:1177–1183. doi:10.1023/A:1025092829696
Rubas W, Zezyk N, Grass GM (1993) Comparison of the permeability characterization of a human colonic epithelium (Caco-2) cell line to colon of rabbit, monkey and dog intestine and human drug absorption. Pharm Res 10:113–118. doi:10.1023/A:1018937416447
Sandström R, Karlsson A, Lennernäs H (1998) The absence of stereoselective P-glycoprotein-mediated transport of R/S-verapamil across the rat jejunum. J Pharm Pharmacol 50:729–735
Taipalensuu J, Tornblom H, Lindberg G et al (2001) Correlation of gene expression of ten drug efflux proteins of the ATP-binding cassette transporter family in normal human jejunum and in human intestinal epithelial caco-2 cell monolayers. J Pharmacol Exp Ther 299:164–170. doi:0022-3565/01/2991-164-170
Tang F, Horie K, Borchardt RT (2002) Are MDCK cells transfected with the human MDR1 gene a good model of the human intestinal mucosa? Pharmacol Res 19:773–779. doi:10.1023/A:1016140429238
Teodoro Z, María JCC, Ricardo NM et al (2004) Assessment and modulation of acamprosate intestinal absorption: comparative studies using in situ, in vitro (CACO-2 cell monolayers) and in vivo models. Eur J Pharm Sci 22:347–356. doi:10.1016/j.ejps.2004.04.004
Tran TT, Mittal A, Aldinger T et al (2005) The elementary mass action rate constants of P-gp transport for a confluent monolayer of MDCKII-hMDR1 cells. Biophys J 88:715–738. doi:10.1529/biophysj.104.045633
Traunecker HCL, Stevens MCG, Kerr DJ et al (1999) The acridonecarboxamide GF120918 potently reverses P-glycoprotein-mediated resistance in human sarcoma MES-Dx5 cells. Br J Cancer 81(6):942–951. doi:10.1038/sj.bjc.6690791
Wang BL (2009) The pharmacokinetics and metabolism of Chinese magnoliavine fruit [D], Peking Union Medical College 2009. doi:CNKI:CDMD:1.2009.150733
Yamashita S, Furubayashi T, Kataoka M et al (2000) Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur J Pharm Sci 10:195–204. doi:10.1016/S0928-0987(00)00076-2
Yee S (1997) In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man-fact or myth. Pharm Res 14:763–766. doi:10.1023/A:1012102522787
Zhao YM, Li FR, Yang JX et al (2005a) Effect of phillyrin on the anti-obesity in nutritive obesity mice. J Chin Med Mate 2:124–125. doi:CNKI:SUN:ZYCA.0.2005-02-00M
Zhao YM, Li FR, Yang JX et al (2005b) Study on the reducing blood lipid and antioxidation effects of phillyrin. Nat Prod Res Dev 17:36–38. doi:CNKI:SUN:TRCW.0.2005-02-007
Acknowledgments
We appreciate the assistance of Dr. Wang ling for cell culture technology. This project was supported by China Postdoctoral Science Foundation (No. 20100471662) and PhD Programs Foundation of Ministry of Education of China (No. 20105132120001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, YX., Ye, LH., Jiang, XH. et al. Assessment and modulation of phillyrin absorption by P-gp using Caco-2 cells and MDR1-MDCKII cells. Eur J Drug Metab Pharmacokinet 36, 41–47 (2011). https://doi.org/10.1007/s13318-011-0026-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-011-0026-0