Abstract
Introduction
Postprandial hyperglycemia is independently associated with many adverse complications, while diets with a low glycemic load are beneficial in improving post-meal glucose levels. This study aims to determine if mobile-app-based low-carbohydrate dietary guidance will reduce exposure to postprandial hyperglycemia in adults with prediabetes.
Methods
This single-blind, randomized controlled pilot study included 100 participants (39 men; mean age 53.6 ± 11.9 years) and was performed in the PKU Care CNOOC Hospital, China. The intervention group (n = 57) received low-carbohydrate dietary guidance through a moblie app (CAReNA) for 3 months, while the control group (n = 43) only received health education on a diabetic diet. The primary outcome was change in time of postprandial hyperglycemia between baseline and 3 months.
Results
The study revealed that the mean time in postprandial hyperglycemia (> 7.8 mmol/l [140 mg/dl]) monitored by flash glucose monitoring changed from 3.27 h/day at baseline to 2.34 h/day at 3 months in the intervention group and from 3.08 h/day to 2.96 h/day in the control group, with a between-group difference of − 0.81 h/day (P < 0.05). Fasting plasma glucose and glycated hemoglobin (HbA1c) in the intervention group decreased significantly, although no significant difference was seen between the two groups. Compared with the control group, the intervention group had a significant decrease in anthropometric and body composition measurements as well as triglycerides.
Conclusion
The mobile-app-based low-carbohydrate dietary guidance effectively reduced the time spent in postprandial hyperglycemia in adults with prediabetes. This new type of nutritional management has beneficial effects on people with prediabetes and needs further research.
Clinical Trial Registration
ChiCTR1900024880.
Similar content being viewed by others
Why carry out this study? |
Prior to clinical diabetes, the elevations of postprandial plasma glucose are the first evident metabolic abnormalities. Elevated post-meal plasma glucose levels may be associated with cardiovascular risk and some other adverse outcomes. |
Strong evidence supports the role of dietary treatment provided by dietitians as being effective for managing prediabetes, which can even reverse the progress of diabetes. |
Because of the large population of diabetes in China, the uneven distribution of medical resources and the serious shortage of dietitians, it is particularly difficult to provide one-on-one or face-to-face nutritional guidance to individuals with prediabetes. |
What was learned from the study? |
Time in postprandial hyperglycemia changed from 3.27 h/day at baseline to 2.34 h/day at 3 months in the intervention group and from 3.08 h/day to 2.96 h/day in the control group, with a between-group difference of − 0.81 h/day. |
Compared to the control group, the post-meal blood glucose level at 45 min to 135 min after the main meal was significantly lower in the intervention group (P < 0.05). |
After the intervention, compared to the control group, the HbA1c was significantly lower in the intervention group (P < 0.05). |
The mobile-app-based low-carbohydrate dietary guidance effectively reduced the time spent in postprandial hyperglycemia in adults with prediabetes. |
Introduction
Because of the rapid change in lifestyle in China, diabetes is rapidly become epidemic. The latest national survey reported the prevalence of diabetes among Chinese adults was 12.8% and that of prediabetes was 35.2% [1]. So far, almost 490 million people are pre-diabetic in China. With the increasing prevalence of diabetes and its related economic burden [2], the prevention and treatment of diabetes have moved from simple clinical treatment to tertiary prevention of diabetes. Hence, the pathogenesis of prediabetes has aroused considerable research interest. Prediabetes usually has no obvious signs or symptoms, but it can gradually develop into type 2 diabetes. Prior to clinical diabetes, the elevations of postprandial plasma glucose are the first evident metabolic abnormalities. Elevated post-meal plasma glucose levels may be associated with cardiovascular risk and some other adverse outcomes [3, 4]. It is reported that there has been increasing evidence that poor control of hyperglycemia appears to play a significant role in the development of complications in diabetes, and the postprandial state is an important contributing factor [5]. Therefore, it is very important to pay attention to and reduce postprandial hyperglycemia in people with prediabetes.
The routine recommendations mainly include antidiabetic drugs, nutrition and exercise therapy [6]. However, drugs may be associated with side effects, and exercise can be difficult to maintain [7]. Nutrition therapy is an effective intervention for the management of prediabetes, especially the lifestyle changes based on dietary habits, which has increased dramatically in the US and worldwide over the last 30 years [8]. Strong evidence supports the role of dietary treatment provided by dietitians as being effective for managing prediabetes [9],which can even reverse the progress of diabetes. Among the various macronutrient proportions, a high carbohydrate content is a key risk factor for hyperglycemia since it is the greatest determinant of post-meal glycemia [10]. The American Diabetes Association described that a low-carbohydrate diet is a dietary strategy that refers to total carbohydrate intake of ≤ 130 g/day, supplementing the diet instead with fat or protein [11]. Previous research suggests that low-carbohydrate diets allow significantly better glycemic control and a trend toward greater weight loss [12,13,14], but there is no conclusive evidence for adults with prediabetes in China. Because of the large diabetic population in China, the uneven distribution of medical resources and the serious shortage of dietitians, it is particularly diffcult to provide one-on-one or face-to-face nutritional guidance to individuals with prediabetes. In recent years, however, with the popularity of smart phones, a series of medically related apps has emerged. Hence, it is worth exploring whether the smart phone, as a convenient tool, is suitable for medical treatment.
Thus, based on these findings, we designed a randomized clinical trial to investigate the effectiveness of mobile-app-based low-carbohydrate dietary guidance in adults with prediabetes. We aimed to explore the effect of low-carbohydrate dietary guidance through a mobile app on postprandial hyperglycemia. In addition, we also investigated the impact of this new type of dietary guidance on other physiologic indicators such as anthropometric measurements, body composition and biochemical parameters.
Methods
Study Design and Participants
The trial took place at the Department of Endocrinology, PKU Care CNOOC Hospital, Tianjin, China. All participants in the study had the research aims and objectives explained and were promised confidentiality throughout the study upon receipt of an informed written consent form. The participants in the study were free to withdraw from the program at any time. The study started in May 2019, and data collection was finalized by September 2019. The clinical trial number is ChiCTR1900024880. The PKU Care CNOOC Hospital Institutional Review Board approved this study. Each patient provided written informed consent before participation. The study was conducted in accordance with the Declaration of Helsinki 1964 and its later amendments.
The medical examination center placed an advertisement that enabled subjects to be recruited for the study. Individuals of both sexes, aged 30–80 years, not taking drugs for treatment (including hypoglycemic, hypotensive, lipid-lowering, etc.) and with prediabetes who had the ability to understand informed consent were considered eligible to participate in the study. Subjects with prediabetes with postprandial blood glucose were selected according to the American Diabetes Association [15] and International Diabetes Federation (IDF) [16] criteria, which include the following features: (1) fasting plasma glucose 100 mg/dl (5.6 mmol/l) to 125 mg/dl (6.9 mmol/l), (2) glycated hemoglobin (HbA1c) 5.7–6.4% and (3) postprandial blood glucose > 140 mg/dl (7.8 mmol/l) 1–2 h after ingestion of food.
The exclusion criteria were as follows: refusal to sign the informed consent form, type 1 diabetes, gestational diabetes mellitus, smoking, alcohol abuse (alcoholic hepatitis) and not having an android smartphone or not able to use a mobile app to communicate.
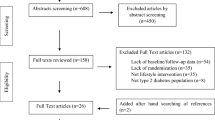
The recruited participants were randomly allocated to either the intervention group or control group by random computer-generated assignment (Fig. 1). The researchers who conducted the randomization procedures were not involved in outcome evaluations and intervention delivery. The researchers involved in outcome evaluation and date analysis were blinded to intervention assignment.
Flash Glucose Monitoring Measurements
A flash glucose monitoring (FreeStyle Libre; Abbott Diabetes Care, Witney, Oxon, UK) sensor was inserted under the skin on the back of the upper arm. The flash glucose monitoring used in this study measured interstitial glucose every 15 min for up to 2 weeks, and the reader retrieved glucose data wirelessly every 8 h. This was a well-tolerated consumer-grade device, and through this device the interstitial glucose measurements were as accurate as capillary blood glucose values [17]. Using FreeStyle Libre software, the glucose data from the flash glucose monitoring can be obtained and stored on a personal computer. In the 14 days preceding the start and end of the study, all participants returned to the hospital to wear the flash glucose monitoring devices. It should be noted that the device locked into masked mode for the 14 days both of the baseline period and end of the study; sensor glucose measurements were not visible to the participants during this time. Post-meal hyperglycemia was defined as a plasma glucose level of 140 mg/dl (7.8 mmol/l) or above 1–2 h after ingestion of food [16]. Participants without postprandial hyperglycemia were excluded before the intervention. We randomly selected the consecutive 7 days of postprandial blood glucose levels after the main meal and observed the fluctuation of post-meal glucose within 3 h (feeding time, 15 min, 30 min, 45 min, 60 min, 75 min, 90 min, 105 min, 120 min, 135 min, 150 min, 165 min, 180 min after the meal).
Use of the Health Management Support Service System
Prior to the study participants needed to use the health management support service system (CAReNA) and download the app (NSD Co., Ltd.). Moreover, professional staff explained how to use the app in detail. The input of necessary items in the app were the meal (including photos), sleep and activity records. Dietary guidance was done through the mobile app by the same dietitian.
Assessment of Anthropometric and Body Composition Measurements
In the beginning of the 1st and 3rd months, the anthropometric parameters of body weight (kg) and body mass index (BMI) were also evaluated. BMI was calculated as weight divided by height squared (kg/m2). Body composition measures included skeletal muscle mass (kg), body fat mass (kg), percentage body fat (percentage) and visceral fat area (cm2) (In-Body720; Biospace Co., Ltd, Seoul, Korea).
Assessment of Biochemical Parameters
Blood samples were taken from overnight fasting at the beginning and end of the interventions, and blood serum was stored at − 80 °C. Biochemical markers [fasting plasma glucose, HbA1c, triglycerides, total cholesterol and high-density lipoprotein cholesterol (HDL cholesterol)] were tested using commercial kits (Labtest, Brazil). Low-density lipoprotein cholesterol (LDL cholesterol) was calculated.
JNCSA Applied Behavioral Analysis for Health Promotion (JABH)
JABH, using a questionnaire with 70 questions, was constructed to analyze diet, exercise, mental health, sleep and health consciousness. It involves eight areas of life behavior including dietary composition/nutrient balance, eating behavior, consumption of sweets and alcoholic drinks, exercise/physical activity, activity volition, stress, fatigue/sleep quality and knowledge of healthy behavior/ability to select health information [18]. Comprehensive assessment is the sum of the eight areas. Higher scores mean better function.
Dietary Assessment Score for Carbohydrates
According to the 14-day diet photos logged into the app, the dietitian judged the nutritional content results (breakfast, lunch and dinner + sugar intake of added meals). The assessment results included four grades: A, B, C and D [A = very good (20–40 g of carbohydrates), B = good (41–60 g of carbohydrates), C = bad (61–80 g of carbohydrates), D = very bad (81 g or above of carbohydrates)]: ten points for A, six points for B, three points for C and one point for D. The total number of occurrences over a 14-day period multiplied by the corresponding score is the dietary assessment score.
Intervention
Randomized participants were provided measurement results of flash glucose monitoring in the first 2 weeks, and the dietitian was required to give instructions for the experiment to the two groups respectively. The dietitian conducted routine health education for adults with prediabetes in the control group [19], instructing participants to record and upload dietary information and photos for each meal instead of dietary guidance. For the intervention group, as the content of initial education, it was necessary to explain the harmful outcomes of post-meal hyperglycemia and the beneficial effects of diets with a low glycemic load in improving postprandial hyperglycemia. In this study, the dietary guidance was mainly focused on a low carbohydrate diet, defined as total carbohydrate intake ≤ 130 g/day [11]. We aimed to have 20–40 g of carbohydrates per meal, 10 g/day in snacks and 70–130 g carbohydrates per day. The intakes of total calories, protein and fat were not limited. The participants had been counseled to choose vegetarian sources of fat and protein and to avoid trans-fatty acids [13]. The methods to manage staple food quantities, selection of foods, amount of food intake, order of eating, speed of eating and condiments were recorded.
In this study, the intervention group received low-carbohydrate dietary guidance twice (dietary guidance I and II) over a 3-month period. Participants in the intervention group used the health management support service system and app, and the dietitian conducted a 2-week low-carbohydrate dietary guidance program (dietary guidance I). Dietary guidance was performed a total of seven times from the next day of the briefing session on the dates of + 1, + 2, + 3, + 4, + 7, + 10, and + 14 days. After 2 weeks, the dietitian would invite the participants in the intervention group to the hospital to give detailed feedback on their performance while they listened to information on the next stage. The second phase of dietary guidance was carried out eight times from the next day of the briefing session at + 1, + 2, + 3, + 4, + 5, + 6, + 7 and +8 weeks (dietary guidance II). The same dietitian evaluated, analyzed and provided photos of diets registered through the health management support service system and provided dietary guidance individually.
Participants in the control group only received early education of diabetes diet management in the briefing session [19], with no low-carbohydrate diet intervention. Two weeks before the end of the study, all participants were asked to return to the hospital to wear the flash glucose monitoring devices and use the app for recording during the last 2 weeks. At the end of this study, we gave the same low-carbohydrate dietary guidance to participants in the control group who wanted to participate.
Outcomes
The primary effectiveness outcome was change in time in postprandial hyperglycemia (> 7.8 mmol/l [140 mg/dl]) monitored by flash glucose monitoring between baseline and 3 months. Sensor-derived glycemic measures comprised: number and duration of postprandial hyperglycemic episodes (sensor glucose > 7.8 mmol/l after meal 1–2 h); episodes defined as at least two consecutive readings, at 15-min intervals, > 7.8 mmol/l; the end of an episode was one reading at or lower than the threshold. We randomly selected 7 consecutive days of postprandial blood glucose values after the main meal and observed the fluctuation of post-meal glucose levels within 3 h. Secondary outcomes were changes in anthropometric measurements, body composition, biochemical parameters, JNCSA applied behavioral analysis for health promotion (JABH) and dietary assessment score.
Statistical Analysis
All the collected data were stored in SPSS version 22.0 (SPSS Inc., Chicago, IL, USA), checked for completeness and tested with the Kolmogorov-Smirnov test for normality. Characteristics of all continuous variables of the subjects at baseline were reported and presented as mean and standard deviation, and classification variables were reported as percentages. Differences in the characteristics at baseline were analyzed using t tests, x2 tests and Kruskal-Wallis rank tests. To examine the postprandial blood glucose changes between groups after the intervention, a two-way (2 × 13) mixed ANOVA was used with group as a between-subject factor (intervention group vs. control group) and time as a within-subject factor (feeding time, 15 min, 30 min, 45 min, 60 min, 75 min, 90 min, 105 min, 120 min, 135 min, 150 min, 165 min, 180 min after the main meal before and after the intervention). Paired t tests were used before and after the intervention within groups. Changes between groups were analyzed using t tests, indicated with 95% confidence limits. The significance was P ≤ 0.05.
Results
A total of 160 participants were assessed for eligibility; 22 subjects were excluded for declining to participate (n = 14) and not having postprandial hyperglycemia (n = 8). One hundred thirty-eight participants were randomly distributed in the intervention group or control group. Twelve individuals in the intervention group dropped out because of lost contact information, loss of motivation or non-compliance with flash glucose monitoring, and 26 subjects were lost to follow-up in the control group. One hundred participants completed the study, 57 in the intervention group and 43 in the control group (Fig. 1). Two groups were comparable in terms of baseline characteristics. The details of the study population and characteristics are listed in Table 1.
Time in postprandial hyperglycemia changed from 3.27 h/day at baseline to 2.34 h/day at 3 months in the intervention group and from 3.08 to 2.96 h/day in the control group, with a between-group difference of –0.81 h/day (P < 0.05). The number of post-meal hyperglycemic events was also significantly reduced in the intervention group (between-group difference, P < 0.05) (Table 2). The two groups were comparable in terms of post-meal glucose levels measured by flash glucose monitoring at baseline (Fig. 2a). Figure 2b shows the comparison of postprandial glucose after the intervention between the two groups, and the differences between groups was significant (P = 0.029). Compared to the control group, the post-meal blood glucose level at 45–135 min after the main meal was significantly lower in the intervention group (P < 0.05).
Changing trends of the postprandial blood glucose levels measured by flash glucose monitoring every 15 min between the two groups at baseline (a) and after the intervention (b). The difference between groups was not significant at baseline (P = 0.133), but was significant after the intervention (P = 0.036). P values are for differences between groups by two-way mixed repeat- measures ANOVA. The post-meal glucose differences at each time point between the two groups were analyzed using t tests. Compared to the control group, the post-meal blood glucose level at 45–135 min after the main meal was significantly lower in the intervention group (*P < 0.05)
At the end of our study, the body weight, BMI, body fat mass, percentage body fat, visceral fat area and all biochemical parameters in the intervention group decreased significantly before and after the intervention (within-group difference, P < 0.05), while the control group had a significant decrease in weight, fasting plasma glucose, HbA1c and HDL cholesterol before and after the intervention. Compared with the control group, the intervention group showed decreased anthropometric and body composition measurements in which the decrease in weight was 1.4 kg (between-group difference, P = 0.001), in BMI was 0.5 kg/m2 (between-group difference, P = 0.006), in body fat mass was 1.6 kg (between-group difference, P < 0.001), in visceral fat area was 9.1 cm2 (between-group difference, P < 0.001) and in triglycerides was 0.36 mmol/l (between-group difference, P = 0.029), respectively (Table 3).
Behavioral analyses for health promotion before and after the intervention in the control and intervention groups are shown in Table 4. Compared to the control group, there were significant increases in two of eight areas of the JABH in the intervention group, in which the increase for eating behavior was 8.0 points (between-group difference, P = 0.013) and for exercise/physical activity was 12.0 points (between-group difference, P = 0.001), respectively. In addition, the dietary assessment score increased significantly in the intervention group compared with the control group (between-group difference, P < 0.001).
Discussion
To our knowledge, no other randomized controlled study has published focused information on mobile-app-based low-carbohydrate dietary guidance in the prevention and treatment of prediabetes. In our study, the mobile-app-based low-carbohydrate dietary guidance significantly reduced the time in postprandial hyperglycemia compared with the control group in people with prediabetes. Moreover, the dietary guidance improved HbA1c and fasting plasma glucose levels in the intervention group, while other results showed significant improvements in the weight, BMI, body fat mass, percentage of body fat, visceral fat area, triglycerides, life behavior assessment and dietary assessment of participants between groups with prediabetes.
Many previous studies have mainly focused on low-carbohydrate diets in diabetes or on glycemic control rather than exploring postprandial hyperglycemia in people with prediabetes. To some extent, the results of our study may have some reference significance. The elevations of postprandial plasma glucose are the first evident metabolic abnormalities proceeding clinical diabetes, possibly due to the decreased insulin sensitivity and consequent decreased suppression of hepatic glucose output after meals due to insulin deficiency [3, 16]. Although the research provided valuable evidence regarding managing postprandial hyperglycemia, the causal association between post-meal blood glucose and complications remained uncertain, and additional study is needed to clarify our understanding of this topic.
Previous studies suggested that a low-carbohydrate diet is an effective method for producing weight loss and may have favorable metabolic effects [13, 14, 20]. In addition, two recent systematic reviews indicated that small improvements in weight loss and/or obesity can prevent the progression from prediabetes to type 2 diabetes over an extended period of time [21, 22]. The results of our study are consistent with several previous studies [9, 23, 24]. Participants' anthropometric values and body composition measurements for weight, BMI and body fat decreased significantly from baseline to 3 months between groups, but the weight and body fat losses were modest. The reason may be that our patients had significantly lower BMI than the subjects in those studies.
Our investigation reported that mean HbA1c in the intervention group decreased from 6.00 to 5.77%. The result was similar to a previous study [25], in which the HbA1c decreased from 5.99 to 5.79%, although conflicting results have been reported. The latest report from Elizabeth et al. suggested that a 3-month low-carbohydrate diet had beneficial effects on HbA1c and fasting plasma glucose [26]. The inconsistent results are likely due to the differences in low-carbohydrate diet interventions. In that study, the low-carbohydrate diet was food-based and low energy, while the intakes of total calories, protein and fat were not limited. Although fasting plasma glucose or HbA1c can be used to define prediabetes, HbA1c has fewer daily changes during stress and illness [27]. Moreover, for every 1% increase in HbA1c, the relative risk of cardiovascular disease increased by 1.2 [25]. Reducing HbA1c levels has become the target of glycemia treatment and the center of its clinical management. The reason why the fasting plasma glucose and HbA1c did not decrease significantly may be that the participants in this study were all prediabetic, and the blood glucose level was not pathologically high, so it was understandable that the decline was only moderate. However, the improvement in the short-term intervention group has already shown benefits, and it is believed that the effect of a prolonged intervention will be more significant. Although our study showed that of the blood parameters only triglycerides had intergroup differences, all indicators in the intervention group decreased significantly before and after the intervention. The results differed slightly from previous studies, likely because of differences in the assessment of prediabetes, in study populations and in ethnic characteristics. Another difference is the the way our dietary guidance was provided, using a mobile app rather than face to face, and at low frequency with only 15 times in 3 months. Greater improvements derive from frequent dietary guidance in metabolic and glycemic control outcomes.
The dietary guidance in our study improved the eating behavior and dietary assessment score, which is consistent with a recent report. Ford et al. [28] demonstrated that dietary intervention decreased the total energy intake and increased the intakes of fruits and vegetables in individuals with prediabetes. Regarding exercise/physical activity, there were no special instructions; in fact, the participants were not supposed to change the way they exercised before the intervention. Surprisingly, exercise/physical activity increased significantly between the groups. The reason may be that the low-carbohydrate diet caused people to pay attention to their health, not only changing their eating habits, but also cultivating a certain exercise habits. Therefore, to some extent the health management support service system also plays a role in promoting healthy lifestyles among participants.
Providing a dietary guidance-based mobile app for adults with prediabetes lacking nutritional knowledge allows for greater engagement of this group because of the flexibility. Developing cost-effective strategies for identifying and managing individuals with overweight/obesity and metabolic abnormalities via screening will have implications for primary care by providing a window of opportunity for early intervention [29]. The 2012 Standards of Medical Care in Diabetes recommended that individuals with prediabetes should receive personalized medical nutrition therapy as needed to achieve their treatment goals, preferably under the guidance of a dietitian nutritionist [30]. Because of China’s large population and the scarcity of specialized dietitians, it is particularly difficult to provide individualized nutritional guidance to adults with prediabetes. To some extent, smart phones, as a convenient tool, played a role in the management of prediabetes in our study. Therefore, compared with traditional medicine, the health management support service system applied as a new medical approach can be used in medical management, especially in the nutritional management of diabetes. In addition, our study provided recommendations for policy and planning actions related to the control of prediabetes screening and interventions.
There were several limitations to our study. Although this was a pilot study, a 3-month intervention is relatively short for drawing strong conclusions or inferring clinical implications. Furthermore, our study was conducted at one clinical site, and the effects of practice styles, participants’ attitudes and characteristics may have influenced the results compared to studies conducted at different sites. In addition, it is possible that the participants who chose to participate felt the greatest need improve their laboratory values, leading to a possible bias.
This study had several strengths, including the randomized controlled trial design and comparisons of low-carbohydrate dietary guidance using a mobile app with regular dietary guidance. This was the first randomized investigation that explored whether the health management support service system improves clinical outcomes in adults with prediabetes. The dietitian continued to monitor food intake and contents during the clinical investigation, while still providing counseling and education. Because dietitian guidance is personalized and has fewer components than other methods, the dietitians can efficiently tailor the interventions to each individual and are less expensive than intensive lifestyle interventions. The health management support service system is also cheaper than intensive lifestyle interventions.
Conclusion
In summary, this study reported that mobile-app-based low-carbohydrate dietary guidance led to significant improvements in postprandial hyperglycemia. Additionally, this low-carbohydrate dietary guidance has a beneficial impact on weight, BMI, body fat mass, percentage of body fat, visceral fat area, triglyceride levels, dietary habits and exercise/physical activity as shown by the JABH and dietary assessment of participants with prediabetes. The inexpensive, accessible health management support service system is very effective, intelligent and efficient in managing a large number of adults with prediabetes. In addition, the intervention showed that not restricting total energy in the low-carbohydrate diets is feasible and practical for practice medical staff to deliver in primary care. Future research must include a longer intervention period with multiple site involvement.
Change history
17 January 2021
In the original article, The corresponding author details were missing. The corresponding author name is Q. Guo and his email address is guoqijp@gmail.com.
References
Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997.
Baena-Diez JM, Penafiel J, Subirana I, et al. Risk of cause-specific death in individuals with diabetes: a competing risks analysis. Diabetes Care. 2016;39:1987–95.
Cavalot F, Petrelli A, Traversa M, et al. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91:813–9.
Hanefeld M, Koehler C, Schaper F, et al. Postprandial plasma glucose is an independent risk factor for increased carotid intima-media thickness in non-diabetic individuals. Atherosclerosis. 1999;144:229–35.
Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in type II diabetes: the epidemiological evidence. Diabetologia. 2001;44:2107–14.
Wasserman DH, Wang TJ, Brown NJ. The vasculature in prediabetes. Circ Res. 2018;122:1135–50.
Roberts S, Craig D, Adler A, McPherson K, Greenhalgh T. Economic evaluation of type 2 diabetes prevention programmes: Markov model of low- and high-intensity lifestyle programmes and metformin in participants with different categories of intermediate hyperglycaemia. BMC Med. 2018;16:16.
Collaboration NCDRF. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–30.
Early KB, Stanley K. Position of the academy of nutrition and dietetics: the role of medical nutrition therapy and registered dietitian nutritionists in the prevention and treatment of prediabetes and type 2 diabetes. J Acad Nutr Diet. 2018;118:343–53.
Sheard NF, Clark NG, Brand-Miller JC, et al. Dietary carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the american diabetes association. Diabetes Care. 2004;27:2266–71.
Turton JL, Raab R, Rooney KB. Low-carbohydrate diets for type 1 diabetes mellitus: a systematic review. PLoS One. 2018;13:e0194987.
Saslow LR, Daubenmier JJ, Moskowitz JT, et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr Diabetes. 2017;7:304.
Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–41.
Forsythe CE, Phinney SD, Fernandez ML, et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids. 2008;43:65–77.
American Diabetes A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13–27.
International Diabetes Federation Guideline Development G. Guideline for management of postmeal glucose in diabetes. Diabetes Res Clin Pract. 2014;103:256–68.
Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17:787–94.
http://www.j-nutrition.or.jp/. Accessed 1 July 2019
Krejci H, Vyjidak J, Kohutiar M. Low-carbohydrate diet in diabetes mellitus treatment. Vnitr Lek. 2018;64:742–52.
Nordmann AJ, Nordmann A, Briel M, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285–93.
Sun Y, You W, Almeida F, Estabrooks P, Davy B. The effectiveness and cost of lifestyle interventions including nutrition education for diabetes prevention: a systematic review and meta-analysis. J Acad Nutr Diet. 2017;117(404–421):e436.
Barry E, Roberts S, Oke J, et al. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: systematic review and meta-analysis of screening tests and interventions. BMJ. 2017;356:i6538.
Raynor HA, Davidson PG, Burns H, et al. Medical nutrition therapy and weight loss questions for the evidence analysis library prevention of type 2 diabetes project: systematic reviews. J Acad Nutr Diet. 2017;117:1578–611.
Parker AR, Byham-Gray L, Denmark R, Winkle PJ. The effect of medical nutrition therapy by a registered dietitian nutritionist in patients with prediabetes participating in a randomized controlled clinical research trial. J Acad Nutr Diet. 2014;114:1739–48.
Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–31.
Morris E, Aveyard P, Dyson P, et al. A food-based, low-energy, low-carbohydrate diet for people with type 2 diabetes in primary care: a randomized controlled feasibility trial. Diabetes Obes Metab. 2019;22:512–20.
American Diabetes A. Standards of medical care in diabetes–2010. Diabetes Care. 2010;33(Suppl 1):S11–61.
Ford CN, Weber MB, Staimez LR, et al. Dietary changes in a diabetes prevention intervention among people with prediabetes: the diabetes community lifestyle improvement program trial. Acta Diabetol. 2019;56:197–209.
Coviello JS, Knobf MT, Laclergue S. Assessing and managing metabolic syndrome and cardiovascular risk in midlife women. J Cardiovasc Nurs. 2013;28:147–56.
American Diabetes A. Standards of medical care in diabetes–2012. Diabetes Care. 2012;35(Suppl 1):S11–63.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Xiaoyu Chen and Haihua Su conceived the concept and design of the study; Daisuke Kunii, Kousuke Kudou, Yiyan Zhang and Ying Zhao provided administrative support; Dan Zhang and Yuanyuan Xing provided the study materials or patients; Jiaqi Teng, Zhiqiang Nie and Xinxin Liu collected and assembled the data; Kaijun Niu, Yong Zhao and Qi Guo analyzed and interpreted the data; all authors wrote the manuscript and approved the final version.
Disclosures
Xiaoyu Chen, Haihua Su, Daisuke Kunii, Kousuke Kudou, Yiyan Zhang, Ying Zhao, Dan Zhang, Yuanyuan Xing, Jiaqi Teng, Zhiqiang Nie, Xinxin Liu, Kaijun Niu, Yong Zhao and Qi Guo have nothing to disclose.
Compliance with Ethics Guidelines
The PKU Care CNOOC Hospital Institutional Review Board approved this study. Each patient provided written informed consent before participation. The study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original online version of this article was revised due to missing corresponding author information.
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12765680.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chen, X., Su, H., Kunii, D. et al. The Effects of Mobile-App-Based Low-Carbohydrate Dietary Guidance on Postprandial Hyperglycemia in Adults with Prediabetes. Diabetes Ther 11, 2341–2355 (2020). https://doi.org/10.1007/s13300-020-00906-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-020-00906-x