Abstract
Introduction
Albiglutide, a selective once-weekly glucagon-like peptide-1 receptor agonist, is being developed for the treatment of type 2 diabetes mellitus. Albiglutide’s effect on cardiac repolarization (QTc interval) was assessed in a randomized, double-blind, placebo-controlled, parallel-group study in healthy subjects with a nested crossover comparison for moxifloxacin.
Methods
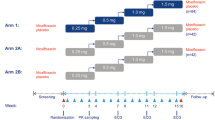
Subjects were randomized to albiglutide (n = 85) or placebo (n = 89) and received injections of 30 mg albiglutide or placebo on Days 1 and 8 and 50 mg albiglutide or placebo on Days 15, 22, 29, and 36. In the placebo group, moxifloxacin was administered on Day −1 in half the subjects and on Day 40 in the other half. Blood samples for albiglutide plasma concentration were drawn on Days 4 and 39 and serial ECGs were extracted from continuous recordings on Days −2 (baseline), −1, 4, 39, and 40.
Results
Demographics were generally similar between albiglutide and placebo subjects: mean age was 29 years and BMI 25 kg/m2. Mean change-from-baseline QTcI (∆QTcI, which was corrected for individual heart rate) on Day 4 after a single dose of albiglutide 30 mg and on Day 39 after repeat dosing with albiglutide 50 mg once weekly was similar to the placebo response. The placebo-corrected ΔQTcI (ΔΔQTcI) on both albiglutide doses was small with the largest ΔΔQTcI of 1.1 ms (upper bound of 90% CI 3.8 ms) on Day 4 and −0.6 ms (upper bound of CI 1.8 ms) on Day 39. Moxifloxacin caused the largest mean effect on ΔΔQTcI of 10.9 ms and the lower bound of the CI was above 5 ms at all preselected timepoints, thereby demonstrating assay sensitivity. Albiglutide was well tolerated and there were no clinically relevant differences in safety data between albiglutide and placebo.
Conclusion
Albiglutide at doses up to 50 mg in healthy subjects did not prolong the QTc interval.
Similar content being viewed by others
Introduction
Albiglutide is a novel glucagon-like peptide-1 (GLP-1) receptor agonist generated through genetic fusion of 2 tandem copies of modified human GLP-1 to human albumin. The GLP-1 sequence has been modified to confer resistance to dipeptidyl peptidase-IV (DPP-IV)-mediated proteolysis. The human albumin moiety of the recombinant fusion protein together with the DPP-IV resistance greatly extends the half-life to ~5 days, allowing once-weekly dosing [1, 2]. Albiglutide retains the glucose-dependent insulinotropic activities of GLP-1 in vitro and in vivo [3] and is being developed for the treatment of type 2 diabetes mellitus (T2DM).
In recent years, some non-antiarrhythmic drugs have been found to delay cardiac repolarization as shown by prolongation of the heart rate-corrected QT interval (QTc) on a standard 12-lead electrocardiogram (ECG) [4]. QTc prolongation can result in life-threatening ventricular proarrhythmias, such as torsade de pointes, which can degenerate into ventricular fibrillation and cause sudden death. As a consequence, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) issued the E14 guidance in 2005 for clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs [5, 6]. This guidance provides recommendations on the design, conduct, analysis, and interpretation of a designated study for the assessment of a new drug’s potential effect on QTc interval, the so-called “thorough QT/QTc (TQT) study”. A TQT study is typically conducted in healthy volunteers and should be placebo-controlled and designed to address potential bias, including the use of randomization and appropriate blinding. A TQT study should also include a positive control to demonstrate that the study is sensitive enough to detect small QTc changes. The vast majority of TQT studies utilize moxifloxacin, a fluoroquinolone antibiotic, for this purpose. Moxifloxacin causes ~7.5–18 ms QTc prolongation [7–9], and specific criteria have been established to confirm “assay sensitivity” with this drug in TQT studies [5].
Based on the long half-life of albiglutide, a parallel design was chosen for this TQT study. To effectively use subjects dosed with placebo or the positive control, a nested crossover comparison was used for demonstration of assay sensitivity [10]. Recently, several TQT studies have used this design [11–14], which is particularly useful for studies with an extended treatment duration, which may be needed to achieve sufficiently high plasma levels of the drug or to improve the tolerability. Gastrointestinal side effects are observed in the GLP-1 receptor class, and in order to minimize the potential for nausea and vomiting, which could complicate the interpretation of a TQT study, subjects in the current study received subcutaneous (SC) albiglutide 30 mg once weekly (or placebo) for the first 2 weeks and then 50 mg once weekly (or placebo) for the remaining 4 weeks. The assessment of a potential QT effect of albiglutide was determined at the maximum intended clinical dose of 50 mg once weekly, which was also the approach used in the TQT studies from others in the GLP-1 receptor class [15, 16].
The objective of the study was to assess the effect of albiglutide 50 mg subcutaneously injected weekly on cardiac repolarization measured as the QTc interval after 6 weeks of treatment.
This study was performed to evaluate whether albiglutide 50 mg subcutaneously injected weekly had an effect on cardiac repolarization in terms of prolongation of the QTc interval, an effect that could cause proarrhythmias in susceptible patients. In line with regulatory expectations, the study was designed to allow exclusion of a QTc effect exceeding the threshold of clinical and regulatory concern (10 ms).
Methods
The study protocol was approved by an institutional review board and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects prior to any study-related procedures.
One hundred and seventy (170) non-smoking male and female healthy adult subjects (18–45 years, inclusive) were planned for enrollment in the study to ensure at least 70 subjects per treatment group. Subjects were to be in good health as judged by the absence of clinically significant diseases or clinically significant abnormal laboratory values. ECG-related exclusion criteria included absence of cardiovascular disease or history of arrhythmias and QTc <450 ms.
Subjects were admitted to the clinical unit on Days −3 and 35 and remained in-house until all procedures were completed on Days 5 and 41, respectively. On Days 1 and 8, subjects received 30 mg SC albiglutide or matching placebo and on Days 15, 22, 29, and 36, subjects received a 50-mg injection of albiglutide or matching placebo. Subjects randomized to albiglutide received moxifloxacin placebo on Days −1 and 40. Moxifloxacin (400 mg oral) was given blindly as a positive control in a nested (crossover) design in the combined albiglutide placebo/moxifloxacin group on Days −1 and 40. Half of these subjects received moxifloxacin 400 mg on Day −1 and moxifloxacin placebo on Day 40 and the other half received moxifloxacin placebo on Day −1 and moxifloxacin 400 mg on Day 40 (Fig. 1). All doses of study medication were administered at approximately 9:00 am.
ECG Methodology
Continuous ECGs were recorded on Days −2, −1, 4, 39, and 40 using M12R ECG 12-lead digital recorders (Global Instrumentation, LLC, Manlius, NY). The continuous 12-lead ECG data were stored on secure digital memory cards and transferred to the central ECG laboratory (iCardiac Technologies, Rochester, NY). ECGs were extracted from the continuous recordings at 1, 2, 3, 6, 12, and 24 h post-dosing. Subjects were resting in the supine or semirecumbent position for at least 15 min before each timepoint from which ECGs were extracted. The ECG assessment was performed on Day 4, representing approximate mean peak plasma level (C max) after a single 30-mg dose and on Day 39, which represents approximate C max after repeat 50-mg doses.
At each timepoint, up to 10 ECG replicates were extracted with TQT Plus, a computer-assisted algorithm utilizing quality and heart rate stability criteria to extract ECG data from continuous 24-h Holter recordings. All readable cardiac cycles from these ECG replicates were assessed for multiple quality metrics, including beat stability, heart rate changes, noise, and other parameters, and were categorized into high- and low-confidence rank. All low-confidence beats were fully reviewed and adjudicated manually by ECG technicians using pass–fail criteria. The beats found acceptable by manual review were included with the high-confidence rank beats in the analysis. ECG interval measurements were made using the High Precision QT measurement technique with lead II as the primary analysis lead. Categorical T-wave morphology analysis and measurement of PR and QRS intervals were performed manually in 3 of the 10 ECG replicates at each timepoint. Final quality control and diagnostic interpretations were performed by the study cardiologist.
Pharmacokinetic Assessment
Blood samples for serial albiglutide pharmacokinetic (PK) assessments were obtained at the same timepoints as ECG assessment on Days −2, −1, 4, 39, and 40. Single blood samples for plasma trough concentrations of albiglutide were also obtained before dosing on Days 8, 15, 22, 29, and 36. Plasma analyses for albiglutide concentrations were performed by GlaxoSmithKline (GSK) Department of Drug Metabolism and PK (King of Prussia, PA) using a chemiluminescent immunoassay. Plasma samples were diluted 100-fold with sample buffer before analysis. Albiglutide was captured using a rabbit anti-human GLP-1 (7–36) amide and detected using a rabbit anti-human serum albumin (HSA) conjugated to biotin. Sample concentrations were determined by interpolation from the standard curve, which was fitted using a weighted (1/x), 4-parameter logistic equation. The validated range of this assay (based on 10 μL of human plasma) is 50–1,500 ng/mL.
Statistical Analysis
The primary endpoint was the placebo-corrected change-from-baseline QTc (∆∆QTc) on Day 39 using the heart rate correction method chosen for the primary analysis, based on prospectively defined criteria [∆∆QTcI (individual correction change-from-baseline formula) or ∆∆QTcF (Fridericia’s correction change-from-baseline formula)]. Secondary ECG endpoints included ∆∆QTc (by the primary method) on Day 4; ∆∆QTc on Day 4 and Day 39 for correction methods not used as primary endpoint; effects on heart rate, PR, and QRS intervals; and changes in T/U-wave morphology and categorical QTc outliers [change-from-baseline QTc (∆QTc) >30 and >60 ms and QTc >450, >480, and >500 ms]. Other endpoints included PK and safety parameters [clinical laboratory evaluations, vital signs, physical examination, and adverse events (AEs)].
The QT corrected for individual heart rate (QTcI) was derived as follows: QT/RR pairs from all six nominal timepoints on Day −2 (baseline) from each subject were used to derive that subject’s individual correction formula. Based on QT/RR pairs from all subjects, the log(RR) coefficient b i was derived from linear mixed-effects modeling: log(QT) = log(a) + b × log(RR/1,000) with gender included as a fixed effect and subject included as a random effect for both intercept and slope. The log(RR) coefficient for each subject, b i , was then used to calculate the QTcI for each subject as follows: \( {\text{QTcI}} = {\text{QT}}\left( {{\text{RR}}/1{,}000} \right)^{{b_{i} }} . \)
To determine which QTc method (QTcF or QTcI) provided the best heart rate correction on treatment, the relationship between QTc (QTcF and QTcI) and the RR interval was investigated using on-treatment data from Day −1 and 40 for moxifloxacin and moxifloxacin placebo and Days 4 and 39 for albiglutide and albiglutide placebo by linear regression modeling: QTc = a + b × RR. The RR coefficient for each subject, b i , was used to calculate the average sum of squared slopes for each of the different QT-RR correction methods and each treatment (moxifloxacin, albiglutide placebo, and albiglutide). The correction method that resulted in the average on-treatment slope closest to 0 for Day 39 albiglutide and albiglutide placebo (the smallest average sum of squared slopes averaged across the 2 treatment groups) was deemed the most appropriate heart rate correction method, as suggested by Tornoe et al. [17], and was therefore to be used as the primary endpoint.
For the primary analysis of ECG effects of albiglutide vs. placebo, time-matched values on Day −2 were used as baseline. For the nested crossover comparison of moxifloxacin vs. moxifloxacin placebo to demonstrate assay sensitivity, the change from baseline was computed based on the corresponding period baseline, i.e., for Day −1, time-matched values on Day −2 were used and for Day 40, values on Day 39 were used as the period baseline. An alternative baseline was also explored for this comparison: For Day −1, Day 40 was used as baseline and for Day 40, Day −1 was the baseline, i.e., using the same duration (40 days) between baseline and assessment as for the primary analysis.
The statistical framework was to demonstrate non-inferiority of albiglutide on QTc as compared with placebo. Statistical analysis of the ECG data was performed using the statistical software R for Windows (Version 2.13.0 or higher). The primary endpoint (∆∆QTc at each timepoint post-dosing) was analyzed using a linear mixed-effects model with fitting terms of treatment, time, and treatment-by-time interaction. Subject was included in the model as a random effect. Baseline QTc was included as a covariate. Replicates at each timepoint were averaged before the analysis. Least-square means and corresponding 90% CIs were constructed at each timepoint. In addition, a similar linear mixed-effects model was repeated with inclusion of gender as a fixed effect. Descriptive statistics were provided for the analysis of heart rate, PR, and QRS intervals.
For the purpose of demonstrating assay sensitivity as described by the ICH E14 Questions & Answers document [5], the contrast in treatment ΔΔQTc = “moxifloxacin-placebo” at 1, 2, and 3 h postdosing was tested against the 1-sided null hypothesis ΔΔQTc <5 ms at the 5% level. Multiplicity was controlled through the Hochberg procedure [18]. If after this procedure, ΔΔQTc was significantly >5 ms for at least 1 timepoint, assay sensitivity was to be considered to have been shown. In addition, 2-sided 90% CIs were obtained for the contrast at all timepoints for descriptive purposes and used in the figures.
The relationship between albiglutide plasma concentrations and the primary endpoint ∆∆QTcI was quantified using a linear mixed-effects modeling approach. Three linear models were explored: (1) a model with an intercept; (2) a model with mean intercept fixed to 0 (with variability); and (3) a model with no intercept. Time-matched concentration was included in the model as covariate and subjects as a random effect for both intercept and slope, whenever applicable. A plot of standardized residuals versus fitted values was used to examine departure from model assumptions. The normal Q–Q plots of the random effects and the within-subject errors were used to investigate the normality of the random effects and the within-subject errors, respectively. A final assessment of the adequacy of the linear mixed-effects model was provided by a goodness-of-fit plot (i.e., the observed concentration quantile-ΔΔQTcI plot) as proposed by Tornoe et al. [17]. Such a plot was used to check the assumption of linearity between albiglutide concentrations and ΔΔQTcI and how well the predicted ΔΔQTcI matched the observed data in the regions of interest.
Results
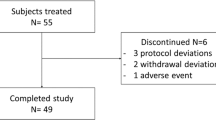
A total of 174 subjects were randomized, of whom 85 were enrolled in the albiglutide group and 89 in the placebo group; with a mean age of 29.5 years (range 18–45 years) and a mean BMI of 25.2 kg/m2 (range 19.3–30.0 kg/m2). ECG parameters at baseline were comparable between treatment groups. With the exception of gender (males, 67% in albiglutide group vs. 50% in placebo group) and race (White/Caucasian/European heritage, 61.2% in albiglutide group and 70.5% in placebo group), demographics and baseline characteristics were generally similar between subjects across treatment groups. Seventy-eight subjects (91.8%) who received albiglutide and 78 subjects (85.4%) who received albiglutide placebo completed the study.
Electrocardiographic Effects of Albiglutide
After repeat dosing with albiglutide 50 mg once weekly, an increase in heart rate of approximately 6–8 bpm was observed (Table 1), whereas a single dose of albiglutide 30 mg was similar to placebo and moxifloxacin.
The slope estimate for QTcI across subjects was somewhat higher [0.347; 90% confidence interval (CI) 0.342, 0.354] than for QTcF (fixed at 0.333). In the comparison between QTcI and QTcF, QTcI consistently resulted in the lowest average sum of squared slopes (SSS) across treatments and was therefore selected as the primary endpoint (Table 2). Albiglutide, when given as a single 30-mg dose or as multiple 50-mg doses given once weekly, did not prolong the QTc interval (Fig. 2). The largest mean ∆∆QTcI was ≤1.1 ms for both doses and the upper bound of the 90% CI was <10 ms at all post-dosing timepoints (Table 3). Results from the analysis of QTcF were entirely consistent with QTcI (data not shown).
When gender was included as an additional fixed effect in the statistical model, results were comparable and conclusions remain the same (data not shown). The study’s ability to demonstrate small QTc changes was confirmed by the moxifloxacin response with a largest mean ΔΔQTcI of 10.9 ms and the lower bound of the 90% CI >5 ms at all prespecified timepoints (1, 2, and 3 h; Table 3). The effect of moxifloxacin on ∆∆QTcI analyzed with the alternative baseline (with 40 days between baseline and moxifloxacin/placebo assessment) showed a similar mean peak effect (13 ms). Despite somewhat wider 90% CIs of the estimate as compared with the period-specific baseline, the lower bound exceeded 5 ms at all prespecified timepoints (data not shown), i.e., the criteria for assay sensitivity were also met using the alternative baseline.
The variability of the QTc measurements calculated as the between-subject SD of ∆QTcI averaged over all timepoints was comparable across treatments: for both albiglutide doses and placebo 7.4 ms, for moxifloxacin 6.1 ms, and for moxifloxacin using the alternative baseline 8.0 ms.
In the PK/QTc analysis, it was demonstrated that a linear model with fixed intercept provided the best fit of the data. A negative relation between albiglutide plasma levels and ΔΔQTcI could be shown with a slope of −0.0003 ms/ng/mL (90% CI −0.0004, −0.0001; P = 0.0008). Using this model, the projected ΔΔQTcI at the geometric mean peak plasma concentration (10,200 ng/mL) could be estimated to −2.51 ms (90% CI −3.65, −1.37; Fig. 3). These results are consistent with the conclusions from the primary analysis that albiglutide at doses ≤50 mg did not prolong the QTc interval.
Goodness-of-fit plot for observed and predicted PK/∆∆QTcI relationship. Red and blue squares with vertical bars denote the observed mean ΔΔQTcI with 90% CI within each plasma concentration decile (i.e., are based on substantially fewer data points as compared with the model); the widths of the CIs are therefore not directly comparable. The solid black line with grey shaded area denotes the model-predicted mean ΔΔQTcI with 90% CI. The horizontal red and blue lines with notches show the range of plasma concentrations within each decile on Days 4 and 39, respectively. CI confidence interval, PK pharmacokinetic, ∆∆QTcI placebo-corrected change from baseline, QTcI individual heart rate-corrected QT interval
There were no subjects with a QTcI value exceeding 450 ms or a ΔQTcI exceeding 30 ms on albiglutide treatment and no treatment-emergent changes in T-wave morphology were noted on albiglutide treatment.
Change-from-baseline PR (ΔPR) was somewhat larger on albiglutide than on placebo, and placebo-corrected, change-from-baseline PR (ΔΔPR) varied between 0.6 and 3.9 ms on albiglutide 30 mg and between 1.5 and 4.5 ms on albiglutide 50 mg. No effect on the QRS interval was observed with ΔΔQRS ≤1.1 ms at all post-dosing timepoints (Table 1).
Safety
Overall, 41.2% of the subjects in the albiglutide group and 39.3% in the albiglutide placebo group reported an AE. The most common AEs reported were nausea, vomiting, and headache (each 8.2%) with albiglutide and headache, nausea (both 11.4%), and contact dermatitis (8.0%) with placebo. Four subjects experienced AEs that led to discontinuation of study drug: asthma (serious AE; albiglutide placebo), maculopapular rash (albiglutide), vomiting (albiglutide placebo), and drug eruption (maculopapular rash due to moxifloxacin). Serious AEs were reported in 2 subjects: acute appendicitis 15 days after the last dose of albiglutide and asthma of moderate severity on Day 1 in a subject in the placebo group who received moxifloxacin on Day −1. Overall evaluations of other laboratory tests, vital sign measurements, ECGs, and physical exams were generally unremarkable; no clinically meaningful differences were observed between the groups.
Discussion
This Phase I, randomized, double-blind, single-center, parallel, nested crossover study investigated the effect of treatment with albiglutide given weekly over 6 weeks compared with albiglutide placebo on cardiac repolarization as determined by the QTc in healthy male and female subjects. Both genders were enrolled into the study since women have longer baseline QTc and may be more susceptible to drug-induced QTc prolongation [19]; if QTc prolongation is observed, there is also a regulatory expectation to analyze the data by gender (see Q&A #8 in [5]). The ICH guidance documents state that a parallel design is recommended for drugs with long elimination half-lives to achieve steady state [6]. In this study, the parallel study design was chosen in light of the long half-life of albiglutide (~5 days) and the need for multiple doses to achieve steady-state concentrations at clinically relevant plasma exposures. This study incorporated an alternative, parallel-study design that incorporated a nested crossover design between the positive control (moxifloxacin) and albiglutide placebo. This design (positive control vs. placebo) provided adequate power for testing the effect of albiglutide and demonstrating assay sensitivity, while decreasing the sample size compared with that needed with a standard 3-arm parallel study design.
Albiglutide did not prolong QT intervals in this study. The QT interval is dependent on the heart rate and a QT correction factor is required to normalize changes in QT interval attributable to heart rate changes. It is currently acknowledged that Bazett’s method for heart rate correction (QTcB) of the QT interval is inappropriate and the reporting of this interval is therefore no longer required in QT assessment studies (Q&A #11 in [5]). The use of QT correction methods, such as QTcB and QTcF formulas, may also introduce errors in estimating drug-induced QT prolongation, secondary to changes in heart rate [20]. Corrections for heart rate using individual subject data and correction factors have been developed and per the ICH guidance are preferred when sufficient QT interval measurements are available for each subject over a range of heart rates [6]. The QTcI applies regression analysis techniques to individual subject pre-therapy QT and RR interval data over a range of heart rates and then applies this correction to on-treatment QT values. Both QTcF and QTcI theoretically correct the QT interval to that which would be observed at a heart rate of 1 cycle per second (60 bpm). In this study, an evaluation of QTcF and QTcI demonstrated that QTcI more appropriately removed the heart rate dependence of the corrected QT interval. The threshold of regulatory concern for a prolonged QT/QTc interval is around 5 ms with an upper bound of the 95% CI that excludes 10 ms [6]. Albiglutide, after a single 30-mg dose and at steady-state concentrations (repeat doses of 50 mg albiglutide), met these criteria.
In the healthy subjects who received multiple doses of 50 mg albiglutide, there was a mean placebo-corrected heart rate between approximately 6 and 8 bpm on Day 39. There were no apparent changes in systolic or diastolic blood pressure. GLP-1 receptor agonists have been reported to increase heart rate in short- and long-term clinical studies [21]. In clinical studies with other approved long-acting GLP-1 agonists (exenatide once weekly and liraglutide), mean increases in heart rate of 4–9 bpm have been observed [22]. An outlier analysis provided additional evidence that albiglutide did not prolong the QT interval. No subjects treated with albiglutide had a QT interval change-from-baseline value >30 ms or a QTcI value >450 ms and no treatment-emergent abnormalities in T-wave morphology were observed. There was no effect on the QRS interval; however, the PR interval increased (~1–5 ms) while on albiglutide treatment.
Moxifloxacin (400 mg) was used as a positive control to validate the ability of the study to detect a change in the corrected QT interval. Assay sensitivity was confirmed by the moxifloxacin response in which the lower bound of the QTc interval was >5 ms at predefined timepoints after a single dose. Assay sensitivity was also confirmed using an alternative baseline to assess the same duration (40 days) between baseline and ECG assessment as for the primary analysis. The observed time-dependent effect on variability was low when using both the period baseline and the alternative baseline with the same duration as for drug assessment. The time-dependent, between-subject variability was lower than what was assumed for sample size calculations (assumed between-subject SD of 12 ms). The observed between-subject variability for both albiglutide doses and placebo and moxifloxacin was <12 ms for both baseline assessments, indicating no gross inadequacies in the sample size for primary endpoints.
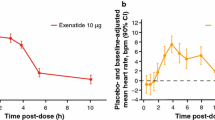
Concentration/QTc modeling demonstrated a negative relationship between albiglutide plasma levels and an effect on ∆∆QTcI; therefore, there was no evidence for QTc prolongation with increasing albiglutide exposures. Consistent with findings for albiglutide, liraglutide also demonstrated no evidence of a dose–response relation with QTc changes [15]. Exenatide showed a weak positive slope of the relationship between QTcF and plasma concentration data [16]; however, a post hoc analysis with QTcI did not show a positive concentration relationship [23]. An infusion of exenatide was utilized to achieve therapeutic and supratherapeutic doses in an additional TQT study [23]; concentration–effect modeling in this study demonstrated no correlation between plasma exenatide concentration and QTcI.
Albiglutide was well tolerated by the healthy subjects in this study. The AE profile suggests that the AEs were unlikely to have impacted the QTcI data. The safety profile for this study was consistent with previous studies with albiglutide conducted in healthy subjects.
A limitation of the study was that albiglutide was tested only at therapeutic doses in this study. The ICH E14 guideline indicates that concentrations higher than those achieved with a therapeutic dose should be tested in a TQT study unless precluded by safety and tolerability due to AEs [6]. Given the occurrence of increased nausea and vomiting at higher doses of albiglutide, the maximal dose in the QT/QTc study was restricted to the maximum therapeutic dose, similar to the dose setting for the QT/QTc studies for liraglutide [15] and exenatide [16]. Furthermore, the concentration/QTc analysis does not support that albiglutide at higher plasma levels would cause QTc prolongation.
In conclusion, albiglutide at clinically relevant doses up to 50 mg in healthy subjects did not prolong the QTc interval. An effect on ∆∆QTcI exceeding the level of regulatory concern (10 ms) could be confidently excluded with both albiglutide doses and there was no evidence of QTc prolongation with increasing albiglutide exposures. Assay sensitivity was confirmed by the moxifloxacin QTc response. These data provide evidence that albiglutide at the proposed clinical doses is not expected to affect cardiac repolarization in subjects with T2DM.
References
Matthews JE, Stewart MW, De Boever EH, et al. Pharmacodynamics, pharmacokinetics, safety, and tolerability of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:4810–7.
Rosenstock J, Reusch J, Bush M, Yang F, Stewart M. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32:1880–6.
Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (Albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53:2492–500.
Darpo B. Spectrum of drugs prolonging the QT interval and the incidence of torsades de pointes. Eur Heart J. 2001;3(Suppl K):70–80.
International Conference on Harmonisation. ICH E14 guideline: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs questions & answers. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Q_As_R1_step4.pdf. Accessed October 22, 2013.
International Conference on Harmonisation. ICH harmonised tripartite guideline. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. Accessed October 22, 2013.
Bloomfield DM, Kost JT, Ghosh K, et al. The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin Pharmacol Ther. 2008;84:475–80.
Darpo B, Nebout T, Sager PT. Clinical evaluation of QT/QTc prolongation and proarrhythmic potential for nonantiarrhythmic drugs: the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use E14 guideline. J Clin Pharmacol. 2006;46:498–507.
Florian JA, Tornoe CW, Brundage R, Parekh A, Garnett CE. Population pharmacokinetic and concentration—QTc models for moxifloxacin: pooled analysis of 20 thorough QT studies. J Clin Pharmacol. 2011;51:1152–62.
Darpo B. The thorough QT/QTc study 4 years after the implementation of the ICH E14 guidance. Br J Pharmacol. 2010;159:49–57.
Graham RA, Chang I, Jin JY, et al. Daily dosing of vismodegib to steady state does not prolong the QTc interval in healthy volunteers. J Cardiovasc Pharmacol. 2013;61:83–9.
Hofmann C, Banken L, Hahn M, Swearingen D, Nagel S, Martin-Facklam M. Evaluation of the effects of bitopertin (RG1678) on cardiac repolarization: a thorough corrected QT study in healthy male volunteers. Clin Ther. 2012;34:2061–71.
Malik M, van Gelderen EM, Lee JH, et al. Proarrhythmic safety of repeat doses of mirabegron in healthy subjects: a randomized, double-blind, placebo-, and active-controlled thorough QT study. Clin Pharmacol Ther. 2012;92:696–706.
Mendzelevski B, Ausma J, Chanter DO, et al. Assessment of the cardiac safety of prucalopride in healthy volunteers: a randomized, double-blind, placebo- and positive-controlled thorough QT study. Br J Clin Pharmacol. 2012;73:203–9.
Chatterjee DJ, Khutoryansky N, Zdravkovic M, Sprenger CR, Litwin JS. Absence of QTc prolongation in a thorough QT study with subcutaneous liraglutide, a once-daily human GLP-1 analog for treatment of type 2 diabetes. J Clin Pharmacol. 2009;49:1353–62.
Linnebjerg H, Seger M, Kothare PA, Hunt T, Wolka AM, Mitchell MI. A thorough QT study to evaluate the effects of single dose exenatide 10 mg on cardiac repolarization in healthy subjects. Int J Clin Pharmacol Ther. 2011;49:594–604.
Tornoe CW, Garnett CE, Wang Y, Florian J, Li M, Gobburu JV. Creation of a knowledge management system for QT analyses. J Clin Pharmacol. 2011;51:1035–42.
Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–8.
Darpo B, Karnad DR, Badilini F, et al. Are women more susceptible than men to drug-induced QT prolongation? Concentration-QTc modeling in a Phase 1 study with oral rac-sotalol. Br J Clin Pharmacol. 2013 Jul 2 [Epub ahead of print]
Indik JH, Pearson EC, Fried K, Woosley RL. Bazett and Fridericia QT correction formulas interfere with measurement of drug-induced changes in QT interval. Heart Rhythm. 2006;3:1003–7.
Sivertsen J, Rosenmeier J, Holst JJ, Vilsboll T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat Rev Cardiol. 2012;9:209–22.
Deacon CF, Marx N. Potential cardiovascular effects of incretin-based therapies. Expert Rev Cardiovasc Ther. 2012;10:337–51.
Darpo B, Sager P, Macconell L, et al. Exenatide at therapeutic and supratherapeutic concentrations does not prolong the QTc interval in healthy subjects. Br J Clin Pharmacol. 2012;74:979–89.
Acknowledgments
Borje Darpo wrote the first draft of this manuscript and critically revised the draft with direction from his coauthors. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data as a whole. All named authors meet the ICMJE criteria for authorship for this manuscript and have given final approval for the version to be published. The authors acknowledge the many contributions to this study from Mark Bush (formerly of GlaxoSmithKline) and Douglas L. Wicks of GlaxoSmithKline for management of manuscript development. Editorial support (copyediting, graphics assistance, and submission assistance) was provided by Diana Talag, ELS, of PharmaWrite, LLC (Princeton, NJ), and funded by GlaxoSmithKline. The study and article processing charges were sponsored by GlaxoSmithKline.
Conflict of interest
Caroline Perry, Rickey R. Reinhardt, Malcolm A. Young, Jessica Matthews and Hui Zhi are employed by GSK and either hold GSK stocks or are eligible to receive or hold GSK stock options. Borje Darpo holds stocks or is eligible to receive or hold stocks from iCardiac Technologies. Meijian Zhou is eligible to receive or hold stocks from iCardiac Technologies.
Compliance with ethics guidelines
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Darpo, B., Zhou, M., Matthews, J. et al. Albiglutide Does Not Prolong QTc Interval in Healthy Subjects: A Thorough ECG Study. Diabetes Ther 5, 141–153 (2014). https://doi.org/10.1007/s13300-014-0055-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-014-0055-1