Abstract
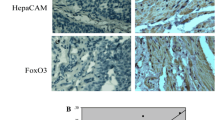
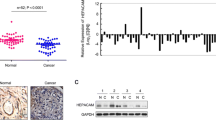
The aim of this study was to explore the correlation between hepatocyte cell adhesion molecule (hepaCAM) and SMAD family member 2/3 (SMAD2/3) in bladder carcinoma, and the involvement of the SMAD2/3 pathway in hepaCAM-induced tumor apoptosis. Immunohistochemistry was used to measure hepaCAM and p-SMAD2/3 protein levels in bladder cancer tissues. Flow cytometry and Hoechst staining were used to study the effect of hepaCAM on cellular apoptosis. Western blot was employed to determine the expression of hepaCAM and SMAD2/3/caspase pathway molecules using a hepaCAM overexpression adenovirus, a caspase inhibitor (Z-VAD-FMK), and a SMAD2/3 activator (transforming growth factor (TGF)-β1), respectively. Translocation of p-SMAD2/3 was measured by immunofluorescence and western blot. HepaCAM proteins were significantly decreased (P < 0.05), while p-SMAD2/3 proteins were remarkably increased (P < 0.05) in bladder carcinoma compared to adjacent tissues. However, the low hepaCAM and high p-SMAD2/3 were not statistically associated with clinicopathological characteristics of the patients. A negative linear correlation between hepaCAM and p-SMAD2/3 was observed according to Pearson analysis (r = −0.712/−0.724, P = 0.008/0.011). Overexpression of hepaCAM activated caspase 3/8/9 and downregulated poly-ADP ribose polymerase (PARP) and p-SMAD2/3. Treatment of bladder cancer cells with Z-VAD-FMK + hepaCAM significantly downregulated procaspase 3/8/9 and PARP and induced cellular apoptosis, compared with that using Z-VAD-FMK alone. Similarly, combined treatment of TGF-β1 + hepaCAM significantly downregulated p-SMAD2/3, procaspase 3/8/9, and PARP and induced apoptosis of bladder cancer cells, compared with TGF-β1 alone. Overexpression of hepaCAM prevented the p-SMAD2/3 translocation from the cytoplasm to the nucleus in bladder cancer cells BIU-87 and T24. Our findings uncover that the p-SMAD2/3 pathway is critical for hepaCAM-induced cancer cell apoptosis and provide valuable insights for current and future Ad-hepaCAM and p-SMAD2/3 clinical trials.





Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. CA Cancer J Clin. 2013;63:11–30.
Babjuk M, Burger M, Zigeuner R. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–53.
Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49.
Parekh DJ, Bochner BH, Dalbagni G. Superficial and muscle-invasive bladder cancer: principles of management for outcomes assessments. J Clin Oncol. 2006;24:5519–27.
Malkowicz SB, van Poppel H, Mickisch G. Muscle-invasive urothelial carcinoma of the bladder. Urology. 2007;69:3–16.
Chung MM, Hoon LL, Shen S. Cloning and characterization of hepaCAM, a novel Ig-like cell adhesion molecule suppressed in human hepatocellular carcinoma. J Hepatol. 2005;42:833–41.
Zhang QL, Luo CL, Wu XH. HepaCAM induces G1 phase arrest and promotes cMyc degradation in human renal cell carcinoma. J Cell Biochem. 2011;112:2910–9.
He YF, Wu XH, Luo CL. Functional significance of the hepaCAM gene in bladder cancer. BMC Cancer. 2010;10:83–90.
Moh MC, Zhang T, Lee LH. Expression of hepaCAM is downregulated in cancers and induces senescence-like growth arrest via a p53/p21-dependent pathway in human breast cancer cells. Carcinogenesis. 2008;29:2298–305.
Tao J, Liu Q, Wu XH. Identification of hypermethylation in hepatocyte cell adhesion molecule gene promoter region in bladder carcinoma. Int J Med Sci. 2013;10:1860–7.
Xu B, He YF, Wu XH. Exploration of the correlations between interferon-gamma in patient serum and HEPACAM in bladder transitional cell carcinoma, and the interferon-gamma mechanism inhibiting BIU-87 proliferation. J Urol. 2012;188:1346–53.
Zhang QL, Luo CL, Yan L. HEPACAM induces apoptosis in renal cell carcinoma 786-0 cell line. Basic Clin Med. 2011;31:73–7.
Vermeulen K, Van Bockstaele DR, Berneman ZN. Apoptosis: mechanisms and relevance in cancer. Ann Hematol. 2005;84:627–39.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2005;144:646–74.
Kehmeier E, Ruhl H, Voland B. Cellular steady-state levels of “high risk” but not “low risk” human papillomavirus (HPV) E6 proteins are increased by inhibition of proteasome-dependent degradation independent of their p53- and E6AP-binding capabilities. Virology. 2011;299:72–87.
Liu Y, Zhang SP, Cai YQ. Cytoprotective effects of selenium on cadmium-induced LLC-PK1 cells apoptosis by activating JNK pathway. Toxicol. 2007;21:677–84.
Mertens‐Talcott SU, Noratto GD, Li X. Betulinic acid decreases ER-negative breast cancer cell growth in vitro and in vivo: role of Sp transcription factors and microRNA-27a: ZBTB10. Mol Carcinog. 2013;52:591–602.
Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–93.
Jones RL, Stoikos C, Findlay JK. TGF-beta superfamily expression and actions in the endometrium and placenta. Reproduction. 2006;132:217–32.
Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–9.
Geng J, Fan J, Ouyang Q. Loss of PPM1A expression enhances invasion and the epithelial-to-mesenchymal transition in bladder cancer by activating the TGF-β/Smad signaling pathway. Oncotarget. 2014;5:5700–11.
Massagué J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–810.
Akhurst RJ, Derynck R. TGF-beta signaling in cancer-a double-edged sword. Trends Cell Biol. 2001;11:S44–51.
Wakefeld LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–9.
Mansouri-Attia N, Tripurani SK, Gokul N. TGFβ signaling promotes juvenile granulosa cell tumorigenesis by suppressing apoptosis. Mol Endocrinol. 2014;28:1887–98.
Wei H, Kamat AM, Aldousari S. Genetic variations in the transforming growth factor beta pathway as predictors of bladder cancer risk. PLoS ONE. 2012;7:e51758.
Schuster N, Krieglstein K. Mechanisms of TGF-mediated apoptosis. Cell Tissue Res. 2002;307:1–14.
Wilkinson DS, Ogden SK, Stratton SA, Piechan JL, Nguyen TT, Smulian GA, et al. A direct intersection between p53 and transforming growth factor beta pathways targets chromatin modification and transcription repression of the alpha-fetoprotein gene. Mol Cell Biol. 2005;25:1200–12.
Du HF, Ou LP, Yang X. A new PKCα/β/TBX3/E-cadherin pathway is involved in PLCε-regulated invasion and migration in human bladder cancer cells. Cell Signal. 2014;26:580–93.
Zhao DD, Lin F, Wu XD. Pseudolaric acid B induces apoptosis via proteasome-mediated Bcl-2 degradation in hormone-refractory prostate cancer DU145 cells. Toxicol in Vitro. 2012;26:595–602.
Wang QJ, Luo CL, Wu XH. HepaCAM and p-mTOR are closely correlated in transitional cell carcinoma of bladder and expression of hepaCAM inhibits proliferation via an AMPK/mTOR-dependent pathway in human bladder cancer cells. J Urol. 2013;190:1912–8.
Xun CH, Luo CL, Wu XH. Expression of hepaCAM and its effect on proliferation of tumor cells in renal cell carcinoma. Urology. 2010;75:828–34.
Song XD, Wang Y, Du HF. Overexpression of HepaCAM inhibits cell viability and motility through suppressing nucleus translocation of androgen receptor and ERK signaling in prostate cancer. Prostate. 2014;74:1023–33.
Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16.
Ruchaud S, Korfali N, Villa P. Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. EMBO J. 2002;21:1967–77.
Olsson M, Zhivotovsky B. Caspases and cancer. Cell Death Differ. 2011;18:1441–9.
Boulares AH, Zoltoski AJ, Contreras FJ. Regulation of DNAS1L3 endonuclease activity by poly(ADP-ribosyl)ation during etoposide-induced apoptosis. Role of poly(ADP-ribose) polymerase-1 cleavage in endonuclease activation. J Bio Chem. 2002;277:372–8.
Wang XR, Yang X, Wang Y. Inducing apoptosis of bladder cancer cell T24 by 5-azacytidine combined with adenovirus-hepaCAM via inhibiting p-AKT. J Chongqing Med Univ. 2014;39:762–7.
Pierreux CE, Nicolas FJ, Hill CS. Transforming growth factor beta-independent shuttling of Smad4 between the cytoplasm and nucleus. Mol Cell Biol. 2000;20:9041–54.
Xu G, Zhong Y, Munir S. Nodal induces apoptosis and inhibits proliferation in human epithelial ovarian cancer cells via activin receptor-like kinase 7. J Clin Endocrinol Metab. 2004;89:5523–34.
Young C, Dae KK, Jashil H. TCEA3 binds to TGF-beta receptor I and induces Smad-independent, JNK-dependent apoptosis in ovarian cancer cells. Cell Signal. 2013;25:1245–51.
Mu XX, Lin S, Yang JH. TGF-b signaling is often attenuated during hepatotumorigenesis, but is retained for the malignancy of hepatocellular carcinoma cells. PLoS ONE. 2013;8:e63436.
Acknowledgments
This work was conducted at the Department of Laboratory Diagnosis, Chongqing Medical University, and is supported by the National Natural Science Foundation of the People’s Republic of China (Grant No. 81072086).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Written informed consent was received from all of the participants. This study was approved by the Ethics Committee of Chongqing Medical University.
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Wang, X., Chen, E., Tang, M. et al. The SMAD2/3 pathway is involved in hepaCAM-induced apoptosis by inhibiting the nuclear translocation of SMAD2/3 in bladder cancer cells. Tumor Biol. 37, 10731–10743 (2016). https://doi.org/10.1007/s13277-016-4821-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4821-8