Abstract
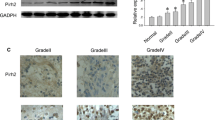
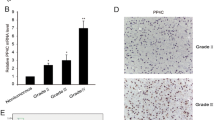
Inhibitor of apoptosis-stimulating protein of p53 (iASPP), encoded by PPP1R13L gene, is often overexpressed in human cancers. From the PPP1R13L gene, at least two isoforms, iASPP-L and iASPP-SV, are produced through alternative splicing. However, the role of these isoforms in glioma is still elusive. In this study, we examined the expression of iASPP-SV in astrocytic glioma tissues with different grades and normal human cerebral tissues. The result showed a higher messenger RNA (mRNA) expression level of iASPP-SV in astrocytic glioma patients with World Health Organization (WHO) grade II to IV in comparison to the normal controls. Additionally, mRNA expression level of iASPP-SV was gradually increased with the raise of the grade in glioma. High mRNA expression level of iASPP-SV was significantly associated with malignant WHO grades (P < 0.001). The protein expression level of iASPP-SV was consistent with the mRNA expression level. The Kaplan–Meier analysis revealed that high iASPP-SV mRNA expression significantly affected overall survival and progression-free survival (both P < 0.001). Furthermore, multivariate analysis indicated that the mRNA expression of iASPP-SV was an independent prognostic marker in glioma (P < 0.001). To further explore the role of iASPP-SV in glioma, U87 cells were transfected with iASPP-SV by lentivirus and then treated with temozolomide (TMZ). Overexpression of iASPP-SV promoted the cell viability and downregulated the expression of pro-apoptosis genes (Bax, Puma, p21, and Noxa) to inhibit apoptosis induced by TMZ. Our study provides the first evidence that high iASPP-SV expression may be a novel prognostic factor and therapeutic target for glioma.




Similar content being viewed by others
References
Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–50.
Rousseau A, Mokhtari K, Duyckaerts C. The 2007 WHO classification of tumors of the central nervous system—what has changed? Curr Opin Neurol. 2008;21:720–7.
Snape TJ, Warr T. Approaches toward improving the prognosis of pediatric patients with glioma: pursuing mutant drug targets with emerging small molecules. Semin Pediatr Neurol. 2015;22:28–34.
Zhang F, Xu CL, Liu CM. Drug delivery strategies to enhance the permeability of the blood–brain barrier for treatment of glioma. Drug Des Devel Ther. 2015;9:2089–100.
Yang QY, Guo CC, Chen ZP. Profile of nimotuzumab in the treatment of high-grade glioma. Oncol Targets Ther. 2015;8:819–25.
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507.
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16:iv1–63.
Bergamaschi D, Samuels Y, O'Neil NJ, Trigiante G, Crook T, Hsieh JK, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet. 2003;33:162–7.
Bergamaschi D, Samuels Y, Jin B, Duraisingham S, Crook T, Lu X. ASPP1 and ASPP2: common activators of p53 family members. Mol Cell Biol. 2004;24:1341–50.
Trigiante G, Lu X. ASPP [corrected] and cancer. Nat Rev Cancer. 2006;6:217–26.
Bell HS, Ryan KM. iASPP inhibition: increased options in targeting the p53 family for cancer therapy. Cancer Res. 2008;68:4959–62.
Sullivan A, Lu X. ASPP: a new family of oncogenes and tumour suppressor genes. Br J Cancer. 2007;96:196–200.
Zhang X, Wang M, Zhou C, Chen S, Wang J. The expression of iASPP in acute leukemias. Leuk Res. 2005;29:179–83.
Liu Z, Zhang X, Huang D, Liu Y, Zhang X, Liu L, et al. Elevated expression of iASPP in head and neck squamous cell carcinoma and its clinical significance. Med Oncol. 2012;29:3381–8.
Cao L, Huang Q, He J, Lu J, Xiong Y. Elevated expression of iASPP correlates with poor prognosis and chemoresistance/radioresistance in FIGO Ib1-IIa squamous cell cervical cancer. Cell Tissue Res. 2013;352:361–9.
Zhang X, Diao S, Rao Q, Xing H, Liu H, Liao X, et al. Identification of a novel isoform of iASPP and its interaction with p53. J Mol Biol. 2007;368:1162–71.
Wang L, Xing H, Tian Z, Peng L, Li Y, Tang K, et al. iASPPsv antagonizes apoptosis induced by chemotherapeutic agents in MCF-7 cells and mouse thymocytes. Biochem Biophys Res Commun. 2012;424:414–20.
Wang JW, Wan XY, Zhu HT, Lu C, Yu WL, Yu CH, et al. Lipotoxic effect of p21 on free fatty acid-induced steatosis in L02 cells. PLoS ONE. 2014;9, e96124.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8.
Liu X, Li F, Zhao S, Luo Y, Kang J, Zhao H, et al. MicroRNA-124-mediated regulation of inhibitory member of apoptosis-stimulating protein of p53 family in experimental stroke. Stroke. 2013;44:1973–80.
Liu X, Zhang Q, Zhang DE, Zhou C, Xing H, Tian Z, et al. Overexpression of an isoform of AML1 in acute leukemia and its potential role in leukemogenesis. Leukemia. 2009;23:739–45.
Zhang WB, Wang Z, Shu F, Jin YH, Liu HY, Wang QJ, et al. Activation of AMP-activated protein kinase by temozolomide contributes to apoptosis in glioblastoma cells via p53 activation and mTORC1 inhibition. J Biol Chem. 2010;285:40461–71.
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8.
Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77.
Inoue K, Fry EA. Alterations of p63 and p73 in human cancers. Subcell Biochem. 2014;85:17–40.
Cai Y, Qiu S, Gao X, Gu SZ, Liu ZJ. iASPP inhibits p53-independent apoptosis by inhibiting transcriptional activity of p63/p73 on promoters of proapoptotic genes. Apoptosis. 2012;17:777–83.
Woodard C, Liao G, Goodwin CR, Hu J, Xie Z, Dos Reis TF, et al. A screen for extracellular signal-regulated kinase-primed glycogen synthase kinase 3 substrates identifies the p53 inhibitor iASPP. J Virol. 2015;89:9232–41.
Lin BL, Xie DY, Xie SB, Xie JQ, Zhang XH, Zhang YF, et al. Down-regulation of iASPP in human hepatocellular carcinoma cells inhibits cell proliferation and tumor growth. Neoplasma. 2011;58:205–10.
Li G, Wang R, Gao J, Deng K, Wei J, Wei Y. RNA interference-mediated silencing of iASPP induces cell proliferation inhibition and G0/G1 cell cycle arrest in U251 human glioblastoma cells. Mol Cell Biochem. 2011;350:193–200.
Liu ZJ, Cai Y, Hou L, Gao X, Xin HM, Lu X, et al. Effect of RNA interference of iASPP on the apoptosis in MCF-7 breast cancer cells. Cancer Invest. 2008;26:878–82.
Morris EV, Cerundolo L, Lu M, Verrill C, Fritzsche F, White MJ, et al. Nuclear iASPP may facilitate prostate cancer progression. Cell Death Dis. 2014;5, e1492.
Kramer D, Schön M, Bayerlová M, Bleckmann A, Schön MP, Zörnig M, et al. A pro-apoptotic function of iASPP by stabilizing p300 and CBP through inhibition of BRMS1 E3 ubiquitin ligase activity. Cell Death Dis. 2015;6, e1634.
Acknowledgments
We thank Prof. Jianxiang Wang (Institute of Hematology and Blood Disease Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College) for providing pCDH-iASPPsv plasmid and the packing plasmids. We thank Lei Wang (Beijing Tiantan Hospital, Capital Medical University), Zhaoke Zheng, and Kaiyuan Song (Beijing Tongren Hospital, Capital Medical University) for collecting some part of tissue samples. This work was supported by the National Natural Science Foundation of China (grant nos. 81000504, 81471209, 81171241), the Beijing Natural Science Foundation (grant no. 7132112), and Special Research Projects in Public Health and Welfare of China (grant no. 20142008-10).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Liu, X., Kang, J., Liu, F. et al. Overexpression of iASPP-SV in glioma is associated with poor prognosis by promoting cell viability and antagonizing apoptosis. Tumor Biol. 37, 6323–6330 (2016). https://doi.org/10.1007/s13277-015-4503-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4503-y