Abstract
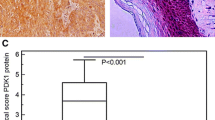
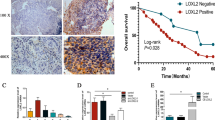
The objectives of the present study are to explore role of pyruvate kinase isoenzyme type M2 (PKM2) in progression of Kazakh’s esophageal squamous cell carcinoma (ESCC) in Xinjiang, China, and to clarify mechanism of PKM2 in malignant phenotype. PKM2 expression was examined using immunohistochemistry (IHC) in 101 matched pairs of ESCC and normal adjacent tissues (NATs) and using enzyme-linked immunosorbent assay (ELISA) in 35 serum samples of Kazakh’s ESCC and 8 serum samples of healthy subjects. To investigate mechanism, small interfering RNA (siRNA)-PKM2 was transfected into ESCC cells. Cell migration and invasion were evaluated by wound healing and Transwell assays. Apoptosis and cell cycle were analyzed by flow cytometry (FCM). PKM2 expression was significantly higher in ESCC tissues (77.2 %, 78/101) compared with matched NAT (P = 0.003) and also higher in serum samples of Kazakh’s ESCC patients (78.84 ng/mL) compared with healthy subjects (13.55 ng/mL) (P = 0.001). Patients with overexpression of PKM2 had a poor prognosis (P = 0.032). After knockdown of PKM2, cell proliferation, migration, and invasion were significantly reduced (P = 0.001), apoptosis increased (P = 0.001), and cell cycle was arrested at G1 phase. PKM2 overexpression was significantly correlated with the worse outcome of Kazakh’s ESCC. Furthermore, PKM2 was involved in progression of ESCC by promoting proliferation and suppressing apoptosis, accelerating invasion, and influencing cell cycle. PKM2 could be a potential biomarker for molecular classification of ESCC.





Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Xu Y, Yu X, Chen Q, Mao W. Neoadjuvant versus adjuvant treatment: which one is better for resectable esophageal squamous cell carcinoma? World J Surg Oncol. 2012;10:173–80.
Zhang SW et al. An analysis of incidence and mortality of esophageal cancer in China, 2003–2007. China Cancer. 2012;21:241–7.
Lambert R, Hainaut P. The multidisciplinary management of gastrointestinal cancer. Epidemiology of oesophagogastric cancer. Best Pract Res Clin Gastroenterol. 2007;21(6):921–45.
Zheng ST, Vuitton L, Sheyhidin I, Vuitton DA, Zhang YM, Lu XM. North Western China: a place to learn more on oesophageal cancer. I. Behavioural and environmental risk factors. Eur J Gastroenterol Hepatol. 2010;22(8):917–25.
Umar SB, Fleischer DE. Esophageal cancer: epidemiology, pathogenesis and prevention. Nat Clin Pract Gastroenterol Hepatol. 2008;5(9):517–26.
Qi YJ, Chao WX, Chiu JF. An overview of esophageal squamous cell carcinoma proteomics. J Proteome. 2012;75:3129–37.
Zhang XH, Liu SQ, Guo CM, Zong JW, Sun MZ. The association of Annexin A2 and cancers. Clin Transl Oncol. 2012;14(9):634–40.
Liu Z, Feng JG, Tuersun A, et al. Proteomic identification of differentially-expressed proteins in esophageal cancer in three ethnic groups in Xinjiang. Mol Biol Rep. 2011;38(5):3261–9.
Christofk HR, Vander Heiden MG, Harris MH, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452(7184):230–3.
Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43(7):969–80.
Lee JM, Kohn EC. Proteomics as a guiding tool for more effective personalized therapy. Ann Oncol. 2010;Suppl 7:205–10.
Matta A, Ralhan R, Desouza LV, et al. Mass spectrometry-based clinical proteomics: head-and-neck cancer biomarkers and drug-targets discovery. Mass Spectrom Rev. 2010;29(6):945–61.
Dunn BK, Wagner PD, Anderson D, et al. Molecular markers for early detection. Semin Oncol. 2010;37(3):224–42.
Minami S, Sato Y, Matsumoto T, et al. Proteomic study of sera from patients with bladder cancer: usefulness of S100A8 and S100A9 proteins. Cancer Genomics Proteomics. 2010;7(4):181–9.
Chong PK, Lee H, Loh MC, et al. Upregulation of plasma C9 protein in gastric cancer patients. Proteomics. 2010;10(18):3210–21.
Vander Heiden MG, Locasale JW, Swanson KD, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329(5998):1492–9.
Shi HS, Li D, Zhang J, et al. Silencing of pkm2 increases the efficacy of docetaxel in human lung cancer xenografts in mice. Cancer Sci. 2010;101(6):1447–53.
Jiang K, He B, Lai L, et al. Cyclosporine A inhibits breast cancer cell growth by down regulating the expression of pyruvate kinase subtype M2. Int J Mol Med. 2012;30(2):302–8.
Landt S, Jeschke S, Koeninger A, et al. Tumor-specific correlation of tumor M2 pyruvate kinase in pre-invasive, invasive and recurrent cervical cancer. Anticancer Res. 2010;30(2):375–81.
Abdullah M, Rani AA, Simadibrata M, et al. The value of fecal tumor M2 pyruvate kinase as a diagnostic tool for colorectal cancer screening. Acta Med Indones. 2012;44(2):94–9.
Eigenbrodt E, Basenau D, Holthusen S, et al. Quantification of tumor type M2 pyruvate kinase (Tu M2-PK) in human carcinomas. Anticancer Res. 1997;17(4B):3153–6.
Yuan Y, Guo-Qing P, Yan T, et al. A study of PKM2, PFK-1, and ANT1 expressions in cervical biopsy tissues in China. Med Oncol. 2012;29(4):2904–10.
Lüftner D, Mesterharm J, Akrivakis C, et al. Tumor type M2 pyruvate kinase expression in advanced breast cancer. Anticancer Res. 2000;20(6D):5077–82.
Roigas J, Schulze G, Raytarowski S, et al. Tumor M2 pyruvate kinase in plasma of patients with urological tumors. Tumour Biol. 2001;22(5):282–5.
Lim JY, Yoon SO, Seol SY, et al. Overexpression of the M2 isoform of pyruvate kinase is an adverse prognostic factor for signet ring cell gastric cancer. World J Gastroenterol. 2012;18(30):4037–43.
Karachaliou N, Papadaki C, Lagoudaki E, et al. Predictive value of BRCA1, ERCC1, ATP7B, PKM2, TOPOI, TOPΟ-IIA, TOPOIIB and C-MYC genes in patients with small cell lung cancer (SCLC) who received first line therapy with cisplatin and etoposide. PLoS One. 2013;8(9):e74611.
Goldberg MS, Sharp PA. Pyruvate kinase M2-specific siRNA induces apoptosis and tumor regression. J Exp Med. 2012;209(2):217–24.
Guo W, Zhang Y, Chen T, et al. Efficacy of RNAi targeting of pyruvate kinase M2 combined with cisplatin in a lung cancer model. J Cancer Res Clin Oncol. 2011;137(1):65–72.
Acknowledgments
This project was supported by the Natural Science Foundation of China (Nos. 81160303, 81260359, 81201891, U1303321) and support from Major Science and Technology Projects of the Xinjiang Uygur Autonomous Region (No. 201430123-1).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary figure 1
Specificity of primary antibody against human PKM2 was evaluated using antigen preadsorption test method. A: primary antibody against human PKM2 was diluted at 1:100, followed by incubation with formalin-fixed paraffin-embeded ESCC section. B: primary antibody against human PKM2 was incubated with the synthetic peptide of PKM2 (from human PKM2 ELISA kit) at 50ug/mL, followed by incubation with formalin-fixed paraffin-embeded ESCC section. The magnification was 200×. (DOC 859 kb)
Supplementary figure 2
Observation of Eca109 cells transfected with siRNA-PKM2 by fluorescence microscopy (Magnification × 100). A: 24 h after transfection with siRNA-PKM2; B. 48 h after transfection with siRNA-PKM2; C. 72 h after transfection with siRNA-PKM2. (DOCX 810 kb)
Supplementary figure 3
Expression of PKM2 mRNA levels in Eca109 after siRNA-PKM2 transfection. Expression level of PKM2 mRNA in Eca109 was significantly down regulated after transfection with siRNA-PKM2 as compared with negative control and untreated cells. *P < 0.05, **P < 0.01 (DOCX 52 kb)
Rights and permissions
About this article
Cite this article
Liu, Q., Liang, M., Liu, T. et al. M2 isoform of pyruvate kinase (PKM2) is upregulated in Kazakh’s ESCC and promotes proliferation and migration of ESCC cells. Tumor Biol. 37, 2665–2672 (2016). https://doi.org/10.1007/s13277-015-4073-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4073-z