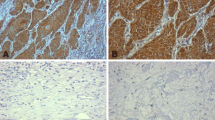
Abstract
Although the expression of tumor necrosis factor receptors (TNFRs) has been associated with clinicopathologic features of some other cancers, their roles in hypopharyngeal squamous cell carcinoma (HPSCC) have not been documented. Forty-five HPSCC specimens were analyzed for the expression of TNFR1 and TNFR2 and its relationship with clinicopathologic factors. Interaction between the two receptors and its effects on TNF-α was investigated by neutralizing TNFR1 and upregulation of TNFR2. The results indicated that, in HPSCC specimens, the expression of TNFR1 but not TNFR2 is associated with clinical staging, T stage, cervical lymph node metastasis, and histologic grade in HPSCC. In Fadu cells, when conjugating with its receptors, TNF-α mediates proliferation effects, and neutralizing TNFR1 and/or upregulating TNFR2 evokes proliferation-inhibiting and apoptosis-inducing effects and potentiates cisplatin (DDP)-induced growth inhibition and apoptosis induction. In conclusion, interaction of TNFR1 with TNFR2 determines the biological characters of HPSCC, and TNFR1 may dominate this process. Moreover, interaction between the two receptors plays important roles in determining the fates of HPSCC cells and thus may serve as a therapeutic target for developing new therapeutic strategies for HPSCC.






Similar content being viewed by others
References
Dou YL, Lin JP, Liu FE, Wang LY, Shu HH, Jiang N, et al. Midazolam inhibits the proliferation of human head and neck squamous carcinoma cells by downregulating p300 expression. Tumor Biol. 2014;35(8):7499–504.
Nishimura H, Sasaki R, Yoshida K, Miyawaki D, Okamoto Y, Kiyota N, et al. Radiotherapy for Stage I or II hypopharyngeal carcinoma. J Radiat Res. 2012;53(6):892–9.
Yu Y, Wang XL, Xu ZG, Fan CC, Li Q. Prognostic value of lymph node ratio in hypopharyngeal squamous cell carcinoma after chemoradiotherapy. Chin Med J (Engl). 2013;126(21):4139–44.
Chu PY, Chang SY. Reconstruction of the hypopharynx after surgical treatment of squamous cell carcinoma. J Chin Med Assoc. 2009;72(7):351–5.
Krstevska V, Stojkovski I, Zafirova-Ivanovska B, Crvenkova S. Prognostic factors in patients with advanced hypopharyngeal squamous cell carcinoma treated with concurrent chemoradiotherapy. J BUON. 2012;17(2):327–36.
Zhu G, Cai G, Liu Y, Tan H, Yu C, Huang M, et al. Quantitative iTRAQ LC-MS/MS proteomics reveals transcription factor crosstalk and regulatory networks in hypopharyngeal squamous cell carcinoma. J Cancer. 2014;5(7):525–36.
Wilson DD, Rahimi AS, Saylor DK, Stelow EB, Jameson MJ, Shonka DC, et al. p16 not a prognostic marker for hypopharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2012;138(6):556–61.
Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992;13(5):151–3.
Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281(5381):1305–8.
Ardestani S, Li B, Deskins DL, Wu H, Massion PP, Young PP. Membrane versus soluble isoforms of TNF-alpha exert opposing effects on tumor growth and survival of tumor-associated myeloid cells. Cancer Res. 2013;73(13):3938–50.
Secchiero P, Gonelli A, Carnevale E, Corallini F, Rizzardi C, Zacchigna S, et al. Evidence for a proangiogenic activity of TNF-related apoptosis-inducing ligand. Neoplasia. 2004;6(4):364–73.
Ziprin P, Ridgway PF, Pfistermuller KL, Peck DH, Darzi AW. ICAM-1 mediated tumor-mesothelial cell adhesion is modulated by IL-6 and TNF-alpha: a potential mechanism by which surgical trauma increases peritoneal metastases. Cell Commun Adhes. 2003;10(2):141–54.
Loetscher H, Pan YC, Lahm HW, Gentz R, Brockhaus M, Tabuchi H, et al. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990;61(2):351–9.
Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278(51):51613–21.
White LE, Santora RJ, Cui Y, Moore FA, Hassoun HT. TNFR1-dependent pulmonary apoptosis during ischemic acute kidney injury. Am J Physiol Lung Cell Mol Physiol. 2012;303(5):L449–459.
Ramesh G, Reeves WB. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiol Ren Physiol. 2003;285(4):F610–618.
Park B, Sung B, Yadav VR, Chaturvedi MM, Aggarwal BB. Triptolide, histone acetyltransferase inhibitor, suppresses growth and chemosensitizes leukemic cells through inhibition of gene expression regulated by TNF-TNFR1-TRADD-TRAF2-NIK-TAK1-IKK pathway. Biochem Pharmacol. 2011;82(9):1134–44.
Ruspi G, Schmidt EM, McCann F, Feldmann M, Williams RO, Stoop AA, et al. TNFR2 increases the sensitivity of ligand-induced activation of the p38 MAPK and NF-κB pathways and signals TRAF2 protein degradation in macrophages. Cell Signal. 2014;26(4):683–90.
Kondo S, Sauder DN. Tumor necrosis factor (TNF) receptor type 1 (p55) is a main mediator for TNF-alpha-induced skin inflammation. Eur J Immunol. 1997;27(7):1713–8.
Dobrzycka B, Terlikowski SJ, Garbowicz M, Niklińska W, Bernaczyk PS, Nikliński J, et al. Tumor necrosis factor-alpha and its receptors in epithelial ovarian cancer. Folia Histochem Cytobiol. 2009;47(4):609–13.
Nakayama S, Yokote T, Tsuji M, Akioka T, Miyoshi T, Hirata Y, et al. TNF-alpha receptor 1 expression predicts poor prognosis of diffuse large b-cell lymphoma, not otherwise specified. Am J Surg Pathol. 2014;38(8):1138–46.
Chiechi A, Novello C, Magagnoli G, Petricoin 3rd EF, Deng J, Benassi MS, et al. Elevated TNFR1 and serotonin in bone metastasis are correlated with poor survival following bone metastasis diagnosis for both carcinoma and sarcoma primary tumors. Clin Cancer Res. 2013;19(9):2473–85.
Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416(6878):345–7.
Jackson-Bernitsas DG, Ichikawa H, Takada Y, Myers JN, Lin XL, Darnay BG, et al. Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates constitutive NF-kappaB activation and proliferation in human head and neck squamous cell carcinoma. Oncogene. 2007;26(10):1385–97.
Isonishi S, Shiotsuka S, Ochiai K, Yasuda M, Terashima Y. Tumor necrosis factor-alpha (TNF alpha) enhances cisplatin cytotoxicity to ovarian carcinoma xenografts. Oncol Rep. 1996;3(6):1049–53.
Benedetti G, Fredriksson L, Herpers B, Meerman J, van de Water B, de Graauw M. TNF-α-mediated NF-κB survival signaling impairment by cisplatin enhances JNK activation allowing synergistic apoptosis of renal proximal tubular cells. Biochem Pharmacol. 2013;85(2):274–86.
Acknowledgments
The research work of this paper is supported by National Natural Science Fund of China (grant number 30340005, 30371521).
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The Publisher and Editor retract this article in accordance with the recommendations of the Committee on Publication Ethics (COPE). After a thorough investigation we have strong reason to believe that the peer review process was compromised.
An erratum to this article is available at http://dx.doi.org/10.1007/s13277-017-5487-6.
About this article
Cite this article
Ma, X., Li, X., Lu, X. et al. RETRACTED ARTICLE: Interaction between TNFR1 and TNFR2 dominates the clinicopathologic features of human hypopharyneal carcinoma. Tumor Biol. 36, 9421–9429 (2015). https://doi.org/10.1007/s13277-015-3684-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3684-8